The videos and information in this post relate to an ECS Journal of Solid State Science and Technology focus issue called: Printable Functional Materials for Electronics and Energy Applications.
(Read/download the focus issue now. It’s entirely free.)
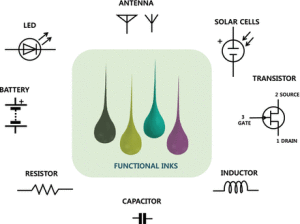
Printing technologies in an atmospheric environment offer the potential for low-cost and materials-efficient alternatives for manufacturing electronics and energy devices such as luminescent displays, thin-film transistors, sensors, thin-film photovoltaics, fuel cells, capacitors, and batteries. Significant progress has been made in the area of printable functional organic and inorganic materials including conductors, semiconductors, and dielectric and luminescent materials.
These new printable functional materials have and will continue to enable exciting advances in printed electronics and energy devices. Some examples are printed amorphous oxide semiconductors, organic conductors and semiconductors, inorganic semiconductor nanomaterials, silicon, chalcogenide semiconductors, ceramics, metals, intercalation compounds, and carbon-based materials.
A special focus issue of the ECS Journal of Solid State Science and Technology was created about the publication of state-of-the-art efforts that address a variety of approaches to printable functional materials and device. This focus issue, consisting of a total of 15 papers, includes both invited and contributed papers reflecting recent achievements in printable functional materials and devices.
The topics of these papers span several key ECS technical areas, including batteries, sensors, fuel cells, carbon nanostructures and devices, electronic and photonic devices, and display materials, devices, and processing. The overall collection of this focus issue covers an impressive scope from fundamental science and engineering of printing process, ink chemistry and ink conversion processes, printed devices, and characterizations to the future outlook for printable functional materials and devices.
The video below demonstrates Printed Metal Oxide Thin-Film Transistors by J. Gorecki, K. Eyerly, C.-H. Choi, and C.-H. Chang, School of Chemical, Biological and Environmental Engineering, Oregon State University.
Step-by-step explanation of the video: