 Topic Close-up #1
Topic Close-up #1
Symposium L06: Nanoporous Materials
Symposium Highlights: Nanoporous materials have unique surface, structural, and bulk properties that enable their applications in electrochemical sensing, catalysis, environmental remediation, photoelectrochemistry, and energy storage. The development of nanoporous materials requires specialized design, synthesis, and characterization methods that tailor pore structure and chemistry to achieve desired material properties. The goal of this symposium is to explore unique challenges and opportunities in the evolution and utilization of nanoporous materials.
Topics of Interest: Including but are not limited to: 1) design, simulation, and/or characterization of nanoporous materials, 2) novel synthesis methods, 3) unique applications of nanoporous materials in electrochemistry and beyond, and 4) insights into the effects of pore structure and surface functionalization on the properties and potential applications of nanoporous materials.


 A new kind of lithium sulfur battery could be more efficient, less expensive, and safer than currently available lithium batteries.
A new kind of lithium sulfur battery could be more efficient, less expensive, and safer than currently available lithium batteries. Ask people to name the most famous historical woman of science and their answer will likely be: Madame Marie Curie. Push further and ask what she did, and they might say it was something related to
Ask people to name the most famous historical woman of science and their answer will likely be: Madame Marie Curie. Push further and ask what she did, and they might say it was something related to  A new sodium-based battery can store the same amount of energy as a state-of-the-art lithium ion at a substantially lower cost.
A new sodium-based battery can store the same amount of energy as a state-of-the-art lithium ion at a substantially lower cost. A new flexible, paper-based supercapacitor could power wearable electronics.
A new flexible, paper-based supercapacitor could power wearable electronics.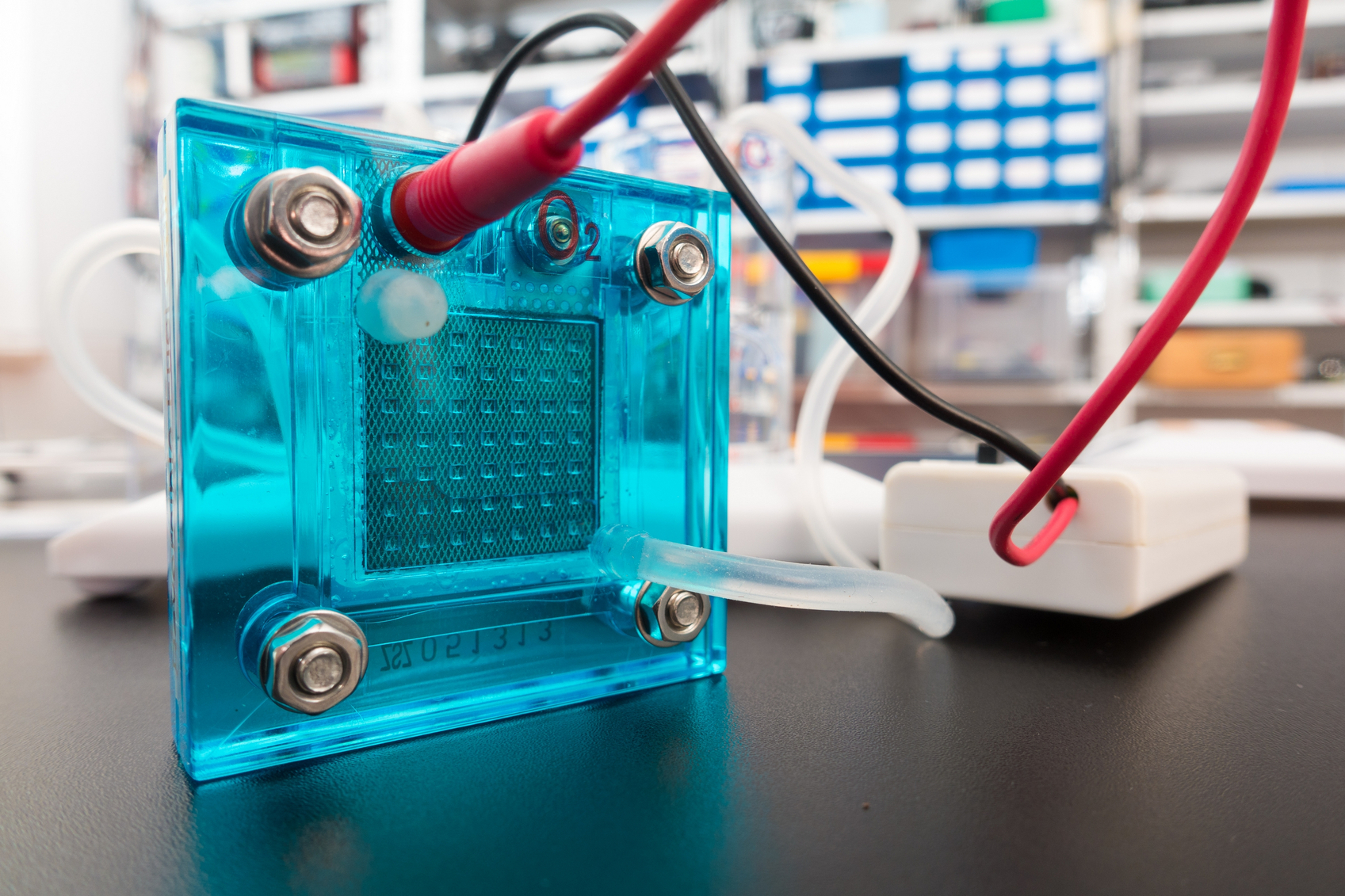 A closer look at catalysts is giving researchers a better sense of how these atom-thick materials produce hydrogen.
A closer look at catalysts is giving researchers a better sense of how these atom-thick materials produce hydrogen. Tech Highlights was prepared by David Enos and Mike Kelly of Sandia National Laboratories, Colm Glynn and David McNulty of University College Cork, Ireland, Zenghe Liu of Verily Life Science, and Donald Pile of Rolled-Ribbon Battery Company. This article was originally published in the
Tech Highlights was prepared by David Enos and Mike Kelly of Sandia National Laboratories, Colm Glynn and David McNulty of University College Cork, Ireland, Zenghe Liu of Verily Life Science, and Donald Pile of Rolled-Ribbon Battery Company. This article was originally published in the  Engineers working to make solar cells more cost effective ended up finding a method for making sonar-like collision avoidance systems in self-driving cars.
Engineers working to make solar cells more cost effective ended up finding a method for making sonar-like collision avoidance systems in self-driving cars.