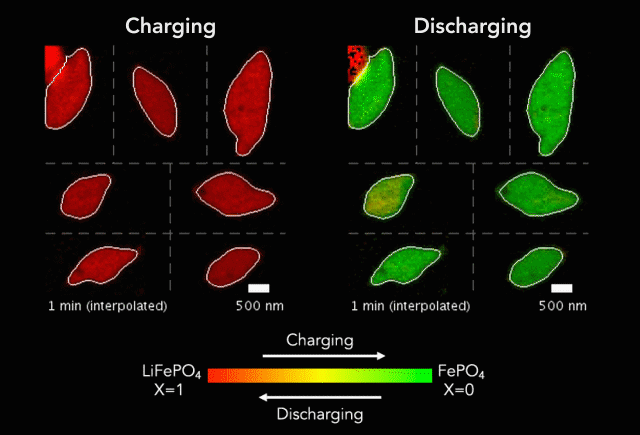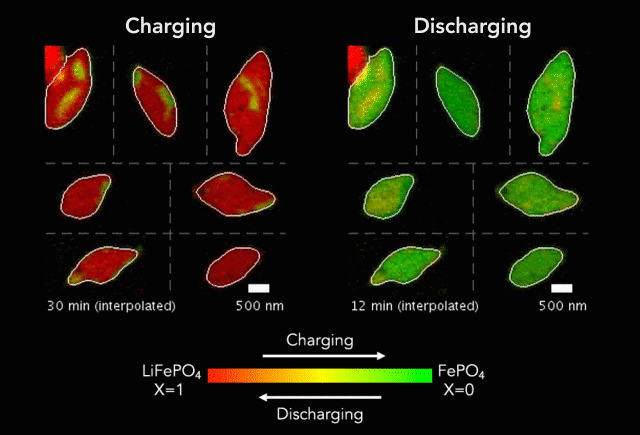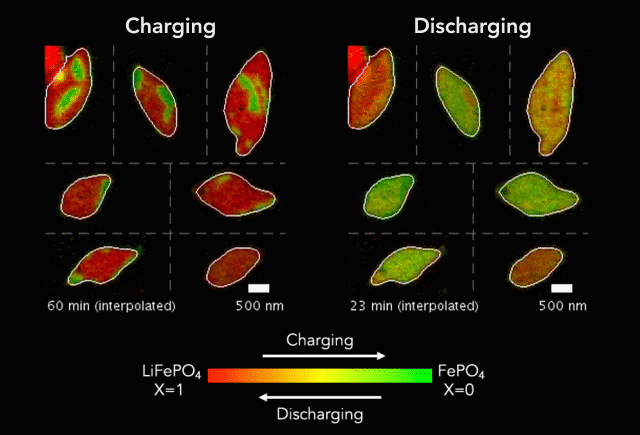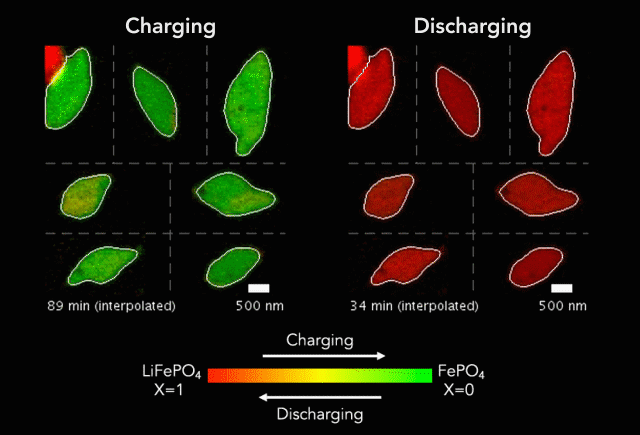
New research shows another step forward in the goal of developing energy storage systems robust enough to store such intermittent sources as wind and solar on a large-scale.
New research shows another step forward in the goal of developing energy storage systems robust enough to store such intermittent sources as wind and solar on a large-scale.
Their work explores the opportunities in solid oxide cells (SOCs), which the group believes to be one of the best prospects in energy storage due to their high efficiency and wide range of scales.
ECS member John Irvine and his team from the University of St. Andrews have set out to overcome traditional barriers in this technology, developing a new method of electrochemical switching to simplify the manufacturing of the electrodes needed to deliver high, long-lasting energy activity.
This from the University of St. Andrews:
The results demonstrate a new way to produce highly active and stable nanostructures – by growing electrode nanoarchitectures under operational conditions. This opens exciting new possibilities for activating or reinvigorating fuel cells during operation.


 The world’s next energy revolution is looming nearer.
The world’s next energy revolution is looming nearer. Electric vehicles have become more visible in the automobile market over the past few years, but many potential buyers still cite one thing as a major deterrent in going electric: range anxiety.
Electric vehicles have become more visible in the automobile market over the past few years, but many potential buyers still cite one thing as a major deterrent in going electric: range anxiety.