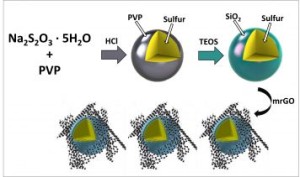
Researchers have investigated a strategy to prevent this “polysulfide shuttling” phenomenon by creating nano-sized sulfur particles, and coating them in silica (SiO2), otherwise known as glass.
Image: Nanoscale
Lithium-sulfur has been a hot topic in battery technology recently. Because of its ability to produce 10 times the amount of energy as a conventional battery, we’ve seen novel innovations such as the all solid state lithium-sulfur battery. Now, the li-sulfur battery is getting a glass coating to further improve its performance.
Researchers at the University of California, Riverside have applied a glass cage-like coating, along with graphene oxide, to the li-sulfur battery. This innovation was developed in order to overcome one of the major issues in commercializing the battery – polysulfides, which cause the battery’s capacity to decrease over its lifetime.
The cathode material traps the polysulfides in a very thin glass cage. Researchers used an organic precursor to construct the trapping barrier.
“Our biggest challenge was to optimize the process to deposit SiO2 – not too thick, not too thin, about the thickness of a virus,” said co-author of the study Mihri Ozkan.
The from University of California, Riverside:
The new generation cathode provided an even more dramatic improvement than the first design, since the team engineered both a polysulfide-trapping barrier and a flexible graphene oxide blanket that harnesses the sulfur and silica together during cycling.
The full paper entitled, “SiO2 – Coated sulfur Particles as a Cathode Material for Lithium-Sulfur Batteries,” is available here.
If you’re interested in lithium-sulfur batteries, make sure to read “All Solid-State Lithium–Sulfur Battery Using a Glass-Type P2S5–Li2S Electrolyte: Benefits on Anode Kinetics.” It’s open access!


