A Stanford University-led team recently published research detailing how particles charge and discharge at the nanoscale, giving new insight into the fundamental functioning of batteries and opening doors for the development of better rechargeables.
This new insight into the electrochemical action that powers Li-ion batteries provides powerful knowledge into the building blocks of batteries.
“It gives us fundamental insights into how batteries work,” says Jongwoo Lim, a co-author of the study. “Previously, most studies investigated the average behavior of the whole battery. Now, we can see and understand how individual battery particles charge and discharge.”
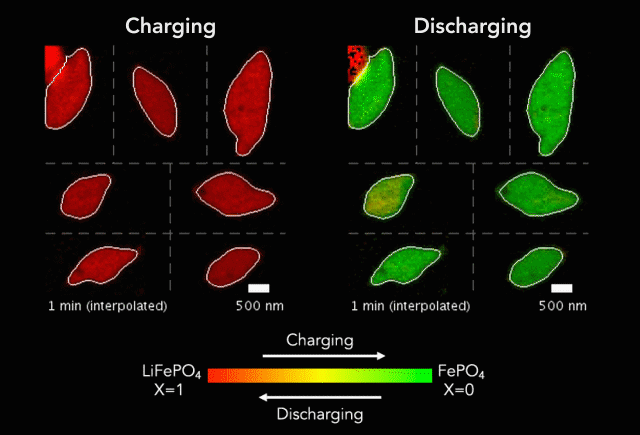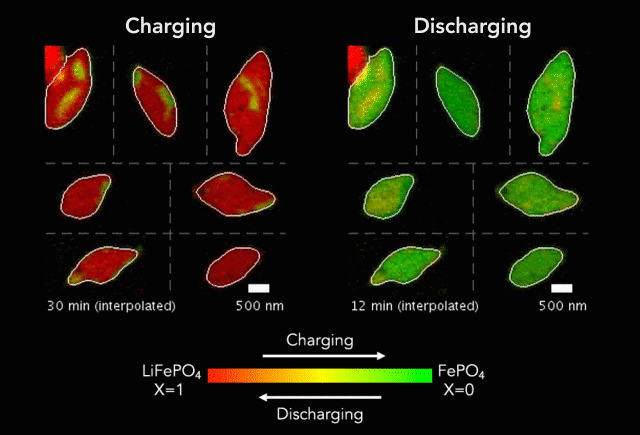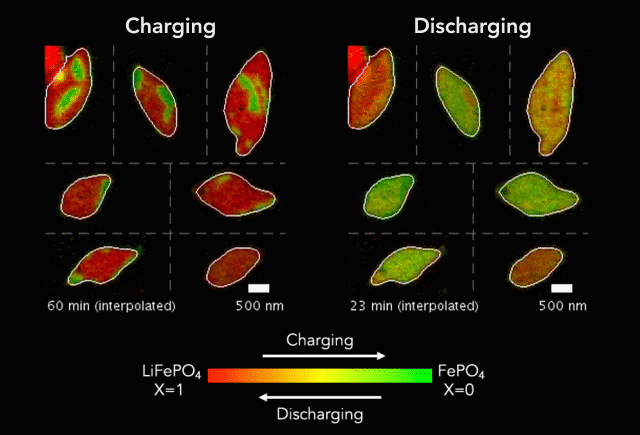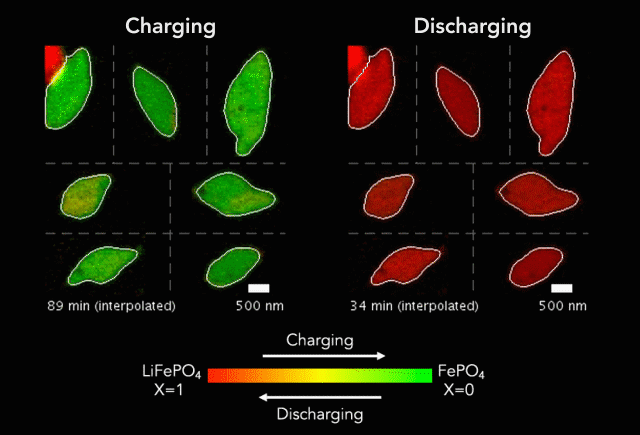
At the heart of every Li-ion battery lies the charge/discharge process. In theory, the ions in the process insert uniformly across the surface of the particles. However, that never happens in practice. Instead, the ions get unevenly distributed, leaving inconsistencies that lead to mechanical stresses and eventually shortened battery life. One way to develop batteries with longer life spans is to understand why these phenomena happens and how to prevent it at the nanoscale.
The recently published research uses x-rays and cutting-edge microscopes to look at this process in real time.
“The phenomenon revealed by this technique, I thought would never be visualized in my lifetime. It’s quite game-changing in the battery field,” says Martin Bazant, co-author of the study.
(MORE: Read “Multicomponent Gas Diffusion in Porous Electrodes,” by Bazant.)
This from Stanford University:
[The team] fashioned a transparent battery using the same active materials as ones found in smartphones and electric vehicles. It was designed and fabricated in collaboration with Hummingbird Scientific. It consists of two very thin, transparent silicon nitride “windows.” The battery electrode, made of a single layer of lithium iron phosphate nanoparticles, sits on the membrane inside the gap between the two windows. A salty fluid, known as an electrolyte, flows in the gap to deliver the lithium ions to the nanoparticles.
Key findings from the study include the newly developed understanding that the charging process is significantly less uniform than the discharging process and that faster charging leads to more uniformity.
“What we’ve learned here is not just how to make a better battery, but offers us a profound new window on the science of electrochemical reactions at the nanoscale,” Bazant says.