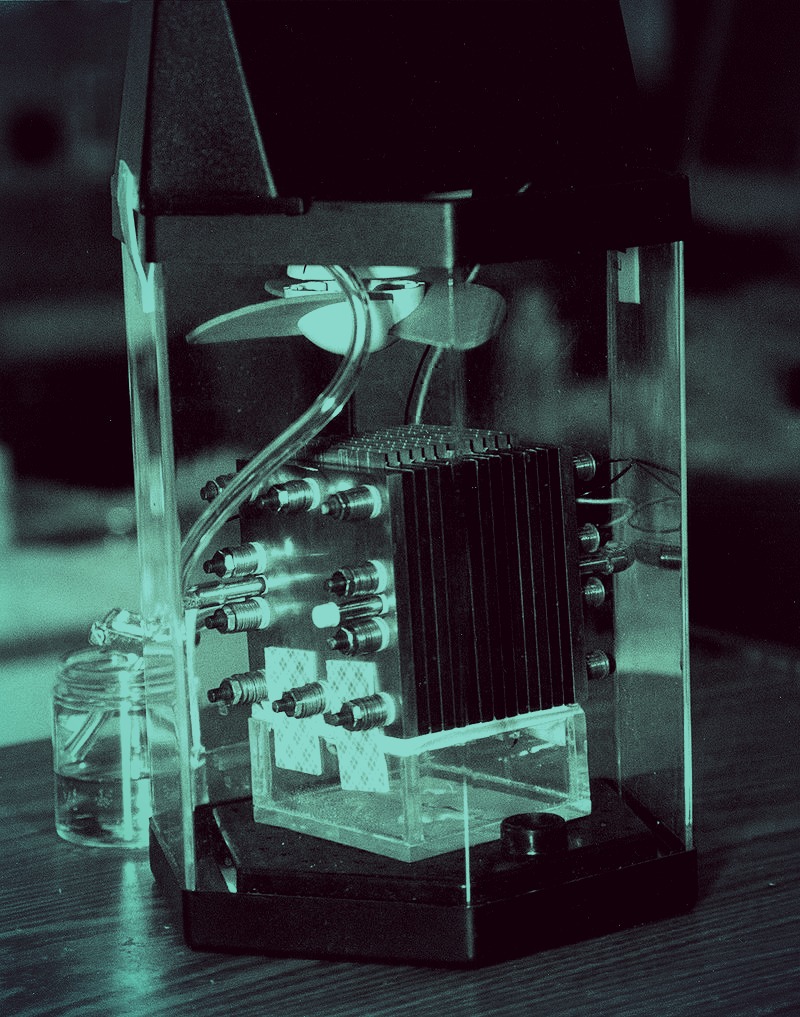
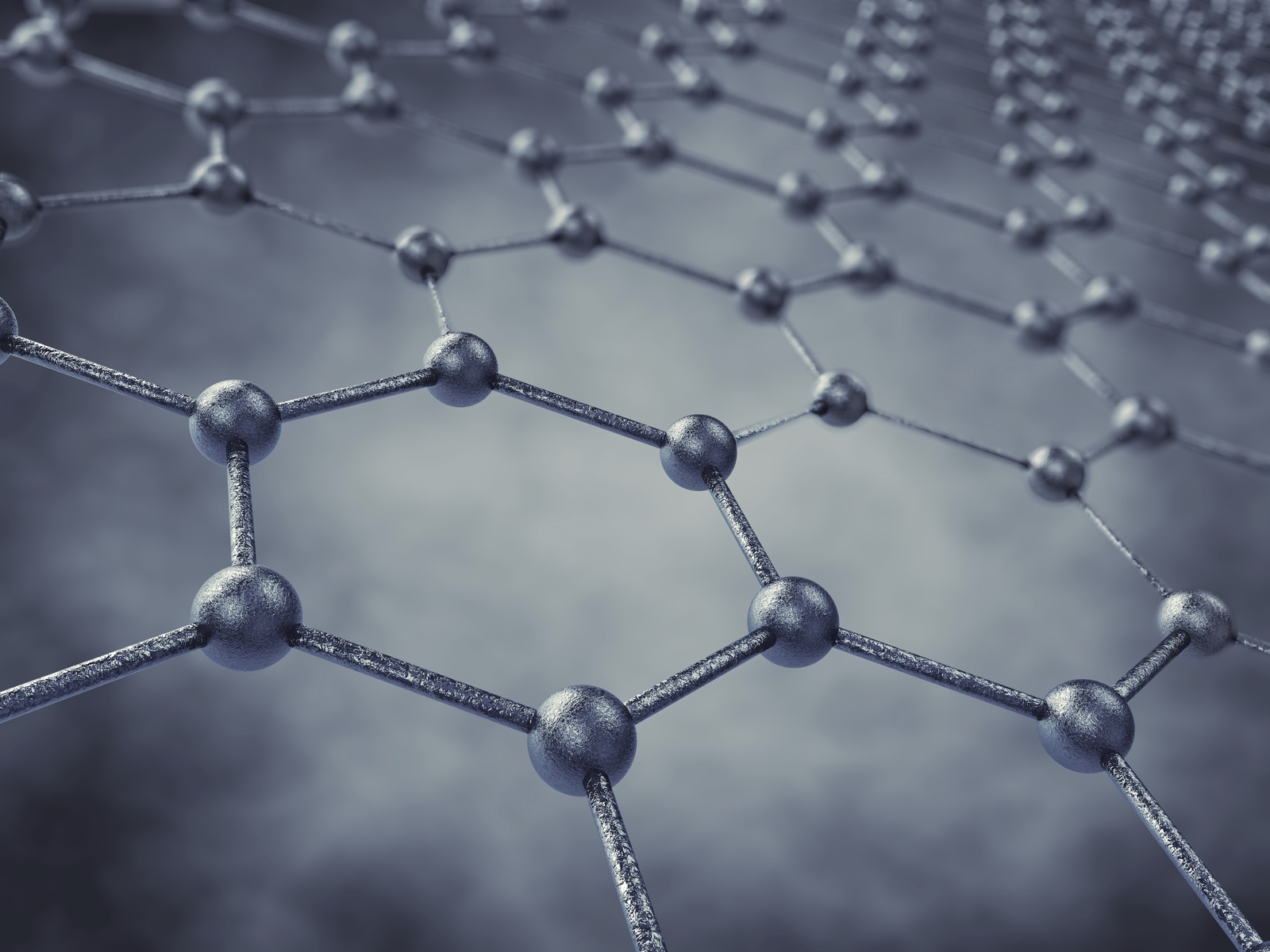
 Scientists have created a durable catalyst for high-performance fuel cells by attaching single ruthenium atoms to graphene.
Scientists have created a durable catalyst for high-performance fuel cells by attaching single ruthenium atoms to graphene.
Catalysts that drive the oxygen reduction reaction that lets fuel cells turn chemical energy into electricity are usually made of platinum, which stands up to the acidic nature of the cell’s charge-carrying electrolyte. But platinum is expensive, and scientists have searched for decades for a suitable replacement.
The ruthenium-graphene combination may fit the bill, says chemist James Tour, a professor of computer science and of materials science and nanoengineering at Rice University, whose lab developed the material. In tests, its performance easily matched that of traditional platinum-based alloys and bested iron and nitrogen-doped graphene, another contender.
“Ruthenium is often a highly active catalyst when fixed between arrays of four nitrogen atoms, yet it is one-tenth the cost of traditional platinum,” Tour says. “And since we are using single atomic sites rather than small particles, there are no buried atoms that cannot react. All the atoms are available for reaction.”


 The
The 
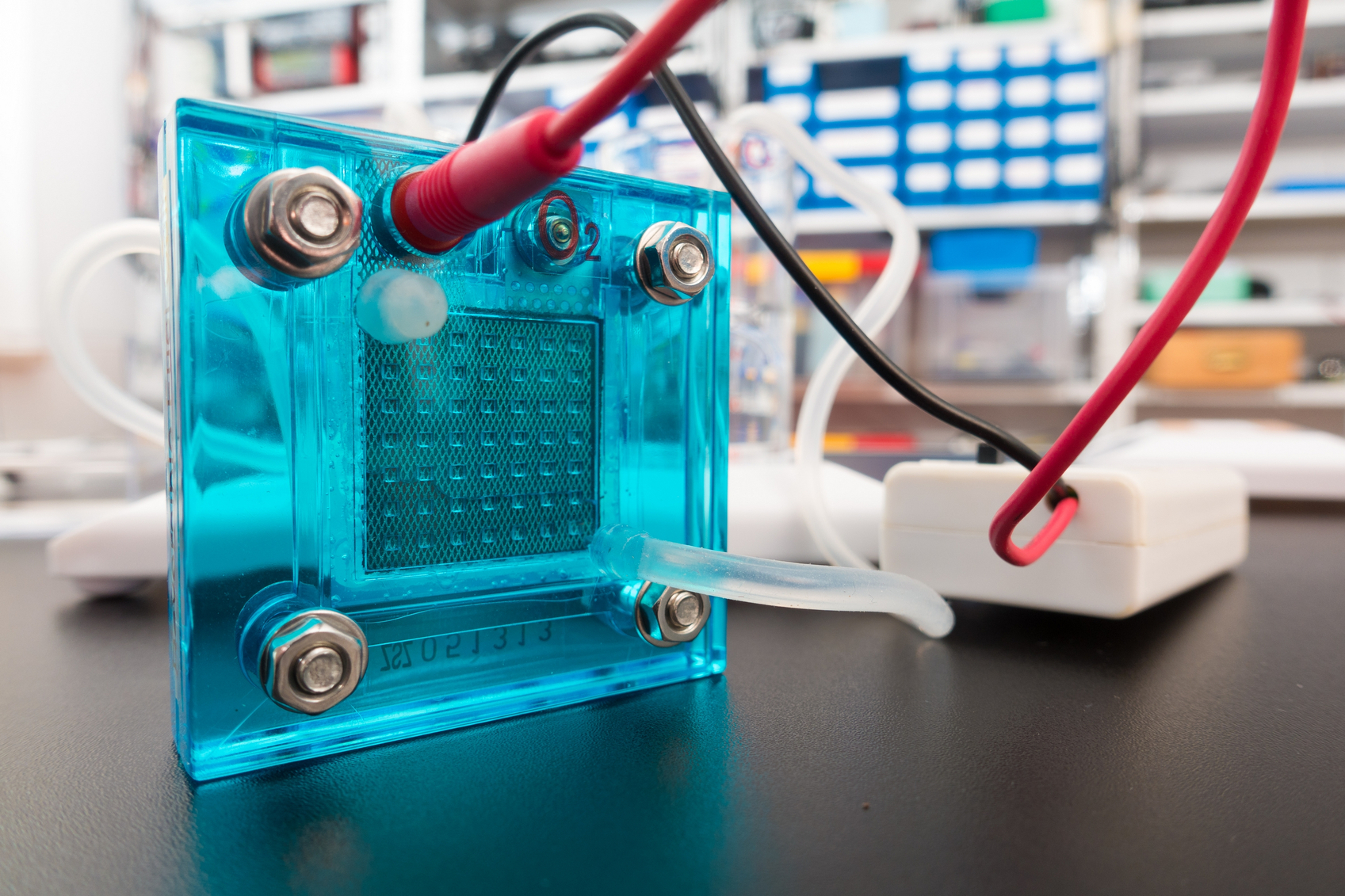 Researchers from Purdue University are making headway on solving issues in electrolyzers and fuel cell development by gaining new insight into electrocatalysts.
Researchers from Purdue University are making headway on solving issues in electrolyzers and fuel cell development by gaining new insight into electrocatalysts. Sometimes the biggest advancements are the smallest in size.
Sometimes the biggest advancements are the smallest in size.