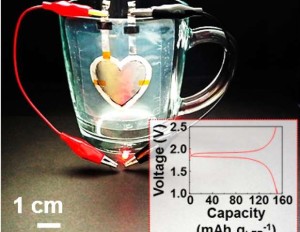
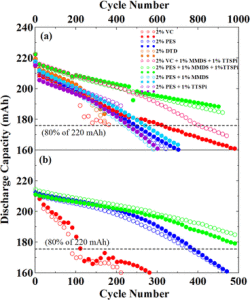
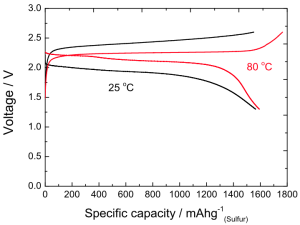
While you may be unfamiliar with Khalil Amine, he has made an immense impact in your life if you happen to use batteries in any way.
As a researcher with a vision of where the science can be applied in the market, Amine has been monumental in developing and moving some of the biggest breakthroughs in battery technology from the lab to the marketplace.
Amine is currently head of the Technology Development Group in the Battery Technology Department at Argonne National Laboratory. From 1998-2008 he was the most cited scientist in the world in the field of battery technology.
He is the chair of the organizing committee for the 18th International Meeting on Lithium Batteries being held this June in Chicago.
Listen to the podcast and download this episode and others for free through the iTunes Store, SoundCloud, or our RSS Feed. You can also find us on Stitcher.