The Journal of The Electrochemical Society is publishing a virtual focus issue in connection with IMLB 2022, hosted in Sydney Australia. This issue is only open to participants of IMLB 2022 who presented therein (invited talks and posters). Review, critical review, perspective, methods, communication, and original research articles are welcomed.
The Journal of The Electrochemical Society is publishing a virtual focus issue in connection with IMLB 2022, hosted in Sydney Australia. This issue is only open to participants of IMLB 2022 who presented therein (invited talks and posters). Review, critical review, perspective, methods, communication, and original research articles are welcomed.
IMLB 2022 is the premier international conference on the state of lithium battery science and technology, as well as current and future related battery systems for application in transportation, industry, grid storage, aviation, aerospace, biomedical, and other promising sectors.
Manuscripts submitted to this issue undergo the normal rigorous review process used for JES, with papers expected to meet the customary high scientific and technical standards for which this journal is known.
(more…)
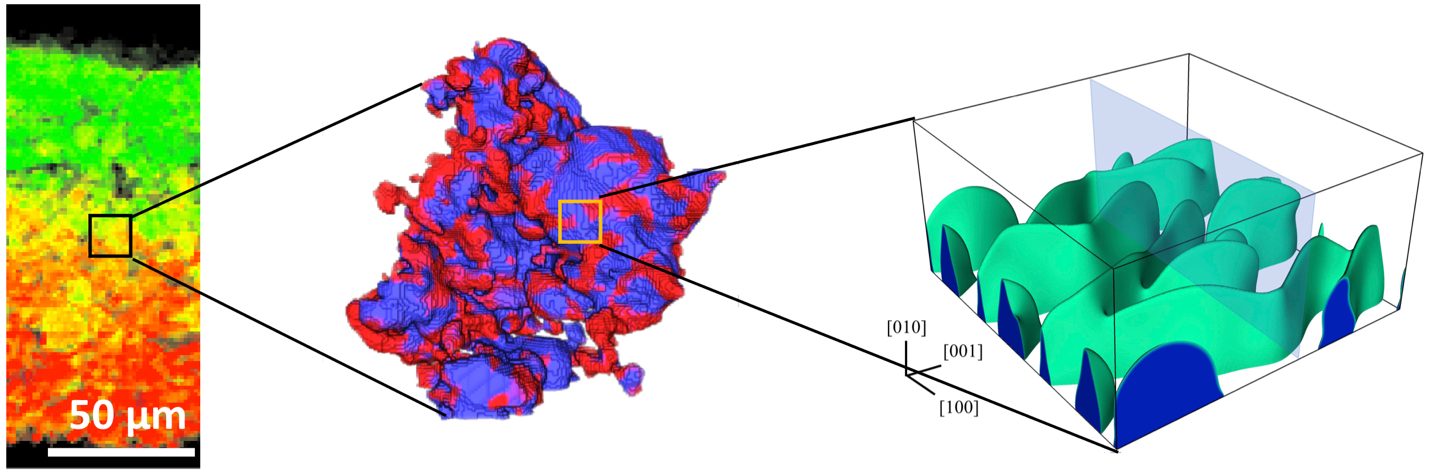
 The Electrochemical Society hosted Dr. Yijin Liu’s live webinar, “A micro-to-nano zoom through a real-world battery with x-ray vision,” on May 17, 2023. Dr. Liu took audience questions during a live Question and Answer session at the end of the presentation. Kindly, he answered, in writing, questions not answered during the broadcast. See his responses below.
The Electrochemical Society hosted Dr. Yijin Liu’s live webinar, “A micro-to-nano zoom through a real-world battery with x-ray vision,” on May 17, 2023. Dr. Liu took audience questions during a live Question and Answer session at the end of the presentation. Kindly, he answered, in writing, questions not answered during the broadcast. See his responses below.