Researchers combine electrochemical investigations with measurement methodologies to develop a new theory to the aging process of lithium ion batteries.
Image: Claudia Niranen/TUM
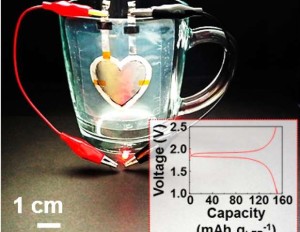
Lithium ion batteries affect everything from small electrical devices to airplanes, yet the battery’s aging process creates limitations to storage capacity. While researchers have not yet been able to determine what causes aging in lithium ion batteries, a research team has made new developments to offer more insight to this downfall and potentially create more youthful batteries.
The study, recently published in the Journal of The Electrochemical Society (JES), describes newly discovered factors that speed up the aging process in lithium ion batteries. This research is especially important in light of efforts in renewable energy, where this energy storage technology could be interwoven with the grid to help bolster efforts in wind and solar.
This from a press release:
The research group determined two key mechanisms for the loss of capacity during operation: The active lithium in the cell is slowly used up in various side reactions and is thus no longer available. The process is very temperature dependent: At 25 °C the effect is relatively weak but becomes quite strong at 60 °C. When charging and discharging cells with a higher upper cut off potential (4.6 V), cell resistance increases rapidly. The transition metals deposited on the anode may increase the conductivity of the pacifying layer and thereby speed up the decomposition of the electrolyte.