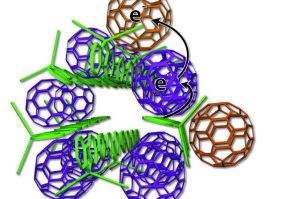
The new arrangement of photovoltaic materials includes bundles of polymer donors (green rods) and neatly organized fullerene acceptors (purple, tan).
Image: UCLA
A team of UCLA scientists are delivering good news on the solar energy front with the development of their new energy storage technology that could change the way scientists think about solar cell design.
Taking a little inspiration from the naturally occurring process of photosynthesis, the researchers devised a new arrangement of solar cell ingredients to make a more efficient cell.
“In photosynthesis, plants that are exposed to sunlight use carefully organized nanoscale structures within their cells to rapidly separate charges — pulling electrons away from the positively charged molecule that is left behind, and keeping positive and negative charges separated. That separation is the key to making the process so efficient,” said Sarah Tolbert, senior author of this research and published ECS author.
PS: Check out Tolbert’s recently published open access paper in the Journal of The Electrochemical Society entitled, “The Development of Pseudocapacitive Properties in Nanosized-MoO2.”
The currently dilemma in solar cell design revolves around developing a product that is both efficient and affordable. While conventional silicon works rather well, it is too expensive to be practical on a large scale. More engineers and researchers have been moving to replace silicon with plastic, but that leads to efficiency levels taking a hit.
“Modern plastic solar cells don’t have well-defined structures like plants do because we never knew how to make them before,” Tolbert said. “But this new system pulls charges apart and keeps them separated for days, or even weeks. Once you make the right structure, you can vastly improve the retention of energy.”
The UCLA team focused on working with two components, which were a polymer donor and a nanoscale fullerene acceptor. These two components work together to absorb sunlight through the polymer donor, which transfers to the fullerene acceptor to produce electrical energy.
ICYMI: We recently sat down with some of the world-leaders in fullerene and nanocarbon research and innovation. Check out how they think this area of science could change the energy infrastructure as we know it.
This from UCLA:
The UCLA technology arranges the elements more neatly — like small bundles of uncooked spaghetti with precisely placed meatballs. Some fullerene meatballs are designed to sit inside the spaghetti bundles, but others are forced to stay on the outside. The fullerenes inside the structure take electrons from the polymers and toss them to the outside fullerene, which can effectively keep the electrons away from the polymer for weeks.
A past member of ECS’s Nanocarbons Division, Yves Rubin, was also a part of this study.
“We don’t have these materials in a real device yet; this is all in solution. When we can put them together and make a closed circuit, then we will really be somewhere,” said Rubin.
For now, the team has shown that inexpensive photovoltaic materials can be arranged to attain a much greater of energy than previously thought.


