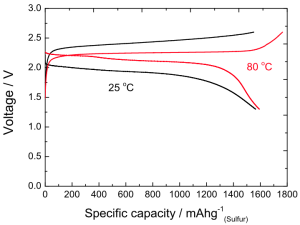
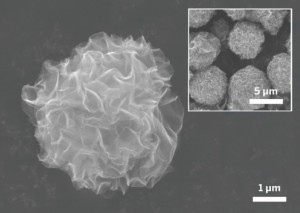
In honor of Alessandro Volta’s 270th birthday, Google is celebrating the man best known for inventing the first battery with today’s Google Doodle.
While Volta was a trained physicist, many consider him to be the first great electrochemist. By inventing the first battery, which he called the electric “pile”, he established the starting point of electrochemical science and technology with the first notable electrochemical storage device.
The turning point for Volta’s development of the battery came in 1780, when his collaborator Luigi Galvani discovered that the contact of two different metals with the muscle of a frog leg resulted in the generation of electric current.
Volta respectfully disagreed with Luigi’s theory that animal tissue was essential in the creation of electricity, arguing that the frog legs served only as an electroscope and further suggested that the true source of stimulation was the contact between dissimilar metals. With this theory, he began experimenting with metals alone in 1794.