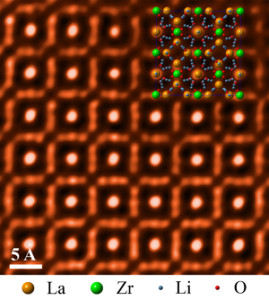
Researcher used microscopy to take an atomic-level look at a cubic garnet material called LLZO that could help enable higher-energy battery designs.
Credit: Oak Ridge National Laboratory
The quest for better batteries is an ongoing trend, and now the researchers from the Department of Energy’s Oak Ridge National Laboratory (ORNL) have yet another development to add.
During their research, the scientists found exceptional properties in a garnet material. They now believe that this could lead to the development of higher-energy battery designs.
This from ORNL:
The ORNL-led team used scanning transmission electron microscopy to take an atomic-level look at a cubic garnet material called LLZO. The researchers found the material to be highly stable in a range of aqueous environments, making the compound a promising component in new battery configurations.
Read the full article here.
While most researcher tend to use a pure lithium anode to improve a battery’s energy density, the ORNL scientists believe the LLZO would be an ideal separator material.
“Many novel batteries adopt these two features [lithium anode and aqueous electrolyte], but if you integrate both into a single battery, a problem arises because the water is very reactive when in direct contact with lithium metal,” said ORNL postdoctoral associate Cheng Ma, first author on the team’s study published in Angewandte Chemie. “The reaction is very violent, which is why you need a protective layer around the lithium.”
With developments such as these, which lead to higher-energy batteries – we begin to improve electrified transportation and electric grid energy storage applications. Due to the importance of higher-energy batteries, researchers tend to explore battery designs beyond the limits of lithium-ion technologies.
Read the full study here.
To find out more about battery and how it will revolutionize the future, check out what the ECS Battery Division is doing. Also, head over to the Digital Library to read the latest research (some is even open access!). While you’re there, don’t forget to sign up for e-Alerts so you can keep up-to-date with the fast-paced world of electrochemical and solid-state science.









 A new kind of lithium sulfur battery could be more efficient, less expensive, and safer than currently available lithium batteries.
A new kind of lithium sulfur battery could be more efficient, less expensive, and safer than currently available lithium batteries.
