Image: CC0
Last week, Samsung ordered a global recall of its Galaxy Note 7 phones after investigations into claims of exploding devices revealed faulty lithium-ion batteries. Now, the FAA is strongly urging passengers to forge bringing the device on airliners due to safety risks.
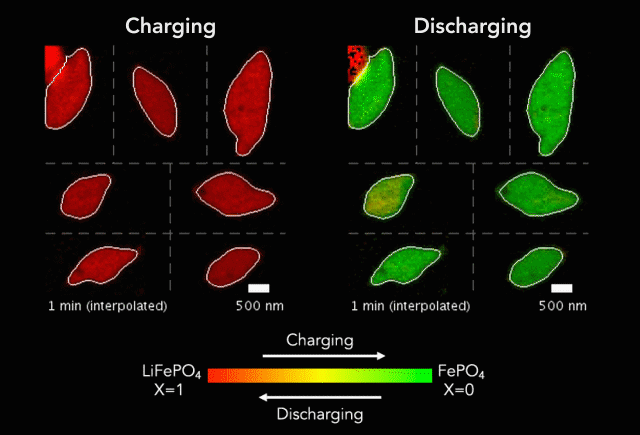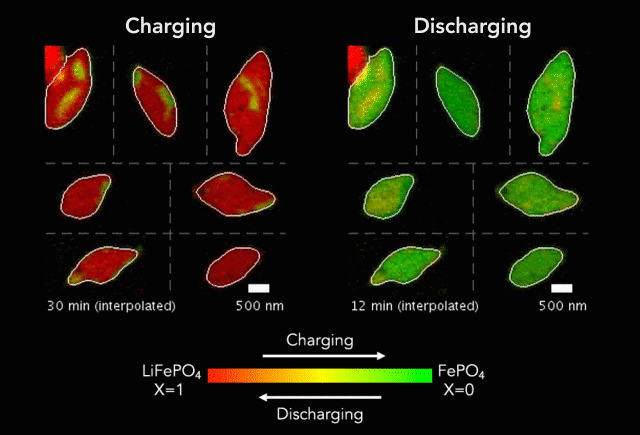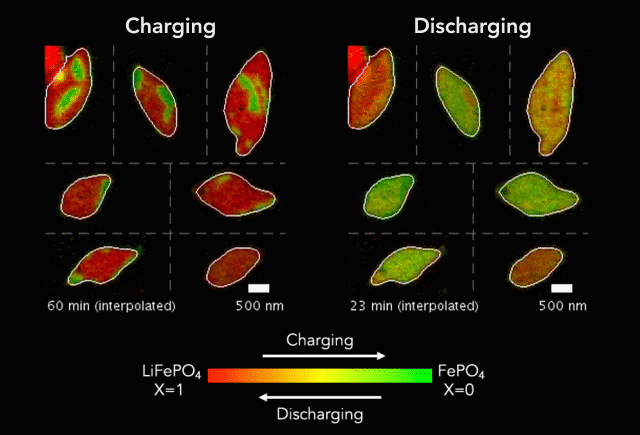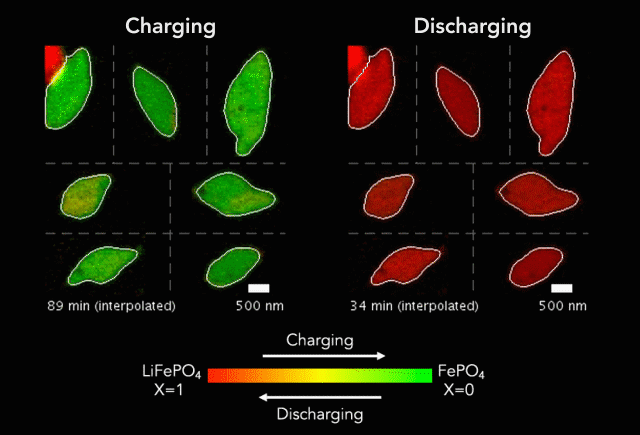
Earlier this year, we spoke to ECS member K.M. Abraham about lithium-ion battery devices and safety concerns associated with them.
“It is safe to say that these well-publicized hazardous events are rooted in the uncontrolled release of the large amount of energy stored in Li-ion batteries as a result of manufacturing defects, inferior active and inactive materials used to build cells and battery packs, substandard manufacturing and quality control practices by a small fraction of cell manufacturers, and user abuses of overcharge and over-discharge, short-circuit, external thermal shocks and violent mechanical impacts,” Abraham said. “Safety hazards of Li-ion batteries occur when the fundamental principle of controlled release of energy on which battery technology is based is compromised by materials and manufacturing defects and operational abuses.”
Read Abraham’s full paper on Li-ion safety and building better batteries.