The following article was originally published in the winter 2017 issue of Interface.
By: Roque J. Calvo, ECS Executive Director
 United States President John F. Kennedy sent a powerful message to the country in his speech at Rice University in1962, “We choose to go to the Moon in this decade and do the other things, not because they are easy, but because they are hard; because that goal will serve to organize and measure the best of our energies and skills, because that challenge is one that we are unwilling to postpone, and one we intend to win.”
United States President John F. Kennedy sent a powerful message to the country in his speech at Rice University in1962, “We choose to go to the Moon in this decade and do the other things, not because they are easy, but because they are hard; because that goal will serve to organize and measure the best of our energies and skills, because that challenge is one that we are unwilling to postpone, and one we intend to win.”
History has shown that it was not necessary to go to the Moon to win Kennedy’s challenge. His primary goal was to elevate U.S. national security during the Cold War when the Soviet Union was advancing as a world power and showing signs of superiority in their space program. The U.S. put a man on the Moon in 1969, but far more important was the spirit of innovation that was created, leading to world-changing new technologies in security, communications, and transportation, which was the true win.
Kennedy understood the importance of innovation and risk taking to the success of a nation and his speech permanently implanted this message into the ideal of science and the role it plays in advancing mankind. He continues to stimulate progress because in his words, “there is new knowledge to be gained … and used for the progress of all people.” It is amazing how Kennedy’s influence has prevailed. In a recent ECS podcast with Steven Chu,* the former U.S. Secretary of Energy and 1997 Nobel Prize winner said, “As a scientist, you better be an optimist … half the science I try to do is really shoot for the Moon.”


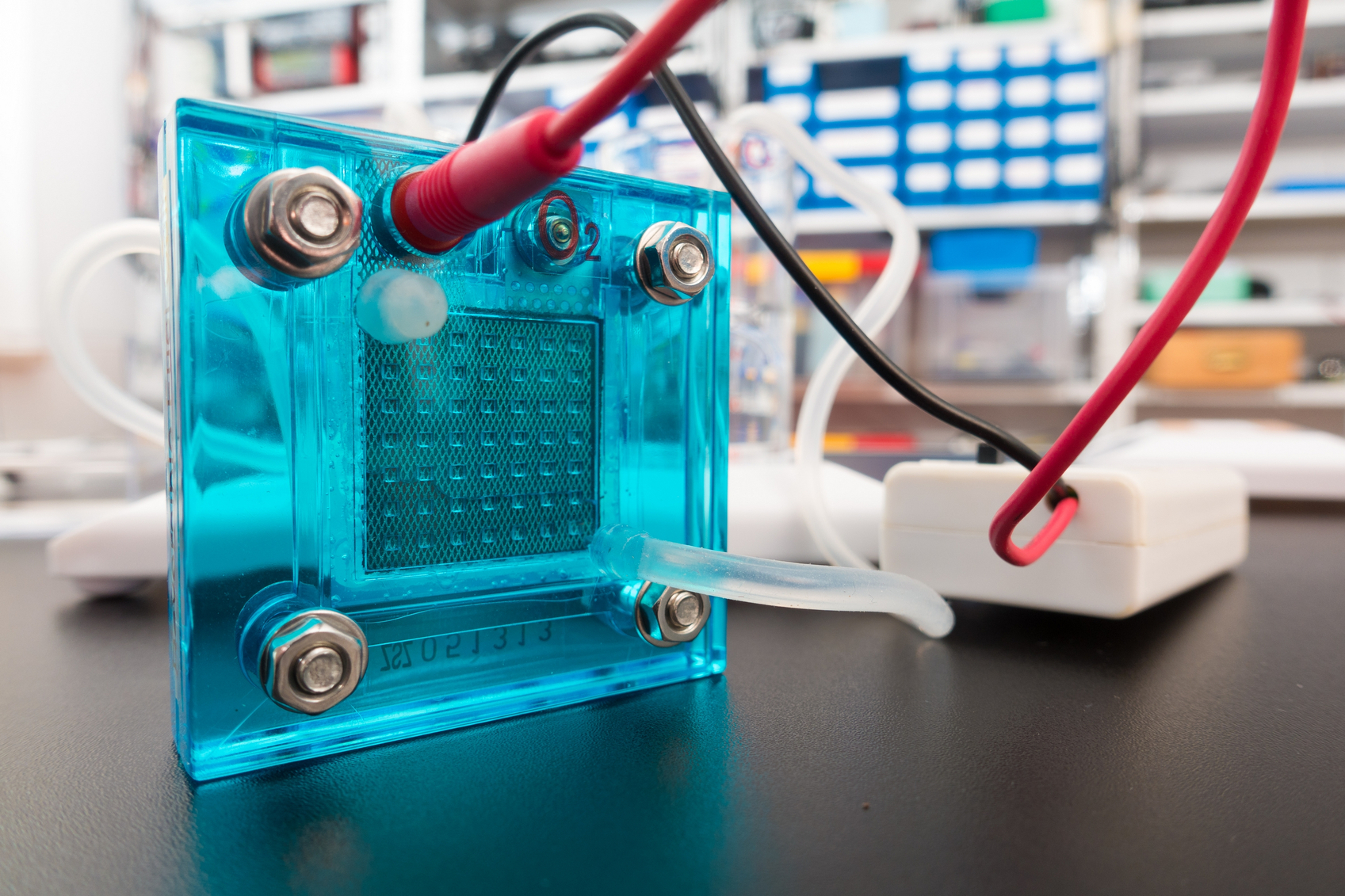 Applying a tiny coating of costly platinum just 1 nanometer thick—about 1/100,000th the width of a human hair—to a core of much cheaper cobalt could bring down the cost of fuel cells.
Applying a tiny coating of costly platinum just 1 nanometer thick—about 1/100,000th the width of a human hair—to a core of much cheaper cobalt could bring down the cost of fuel cells.


 A new issue of ECS Transactions (ECST) has just been published. This issue incorporates 42 papers presented at the 18th International Conference on Advanced Batteries, Accumulators and Fuel Cells (ABAF 2017). This conference was held in Brno, Czech Republic, September 10-13, 2017.
A new issue of ECS Transactions (ECST) has just been published. This issue incorporates 42 papers presented at the 18th International Conference on Advanced Batteries, Accumulators and Fuel Cells (ABAF 2017). This conference was held in Brno, Czech Republic, September 10-13, 2017.