
Image: CC0
Last week, Samsung ordered a global recall of its Galaxy Note 7 phones after investigations into claims of exploding devices revealed faulty lithium-ion batteries. Now, the FAA is strongly urging passengers to forge bringing the device on airliners due to safety risks.
Earlier this year, we spoke to ECS member K.M. Abraham about lithium-ion battery devices and safety concerns associated with them.
“It is safe to say that these well-publicized hazardous events are rooted in the uncontrolled release of the large amount of energy stored in Li-ion batteries as a result of manufacturing defects, inferior active and inactive materials used to build cells and battery packs, substandard manufacturing and quality control practices by a small fraction of cell manufacturers, and user abuses of overcharge and over-discharge, short-circuit, external thermal shocks and violent mechanical impacts,” Abraham said. “Safety hazards of Li-ion batteries occur when the fundamental principle of controlled release of energy on which battery technology is based is compromised by materials and manufacturing defects and operational abuses.”
Read Abraham’s full paper on Li-ion safety and building better batteries.


 Scholarly publishing news has been buzzing about 1science’s
Scholarly publishing news has been buzzing about 1science’s 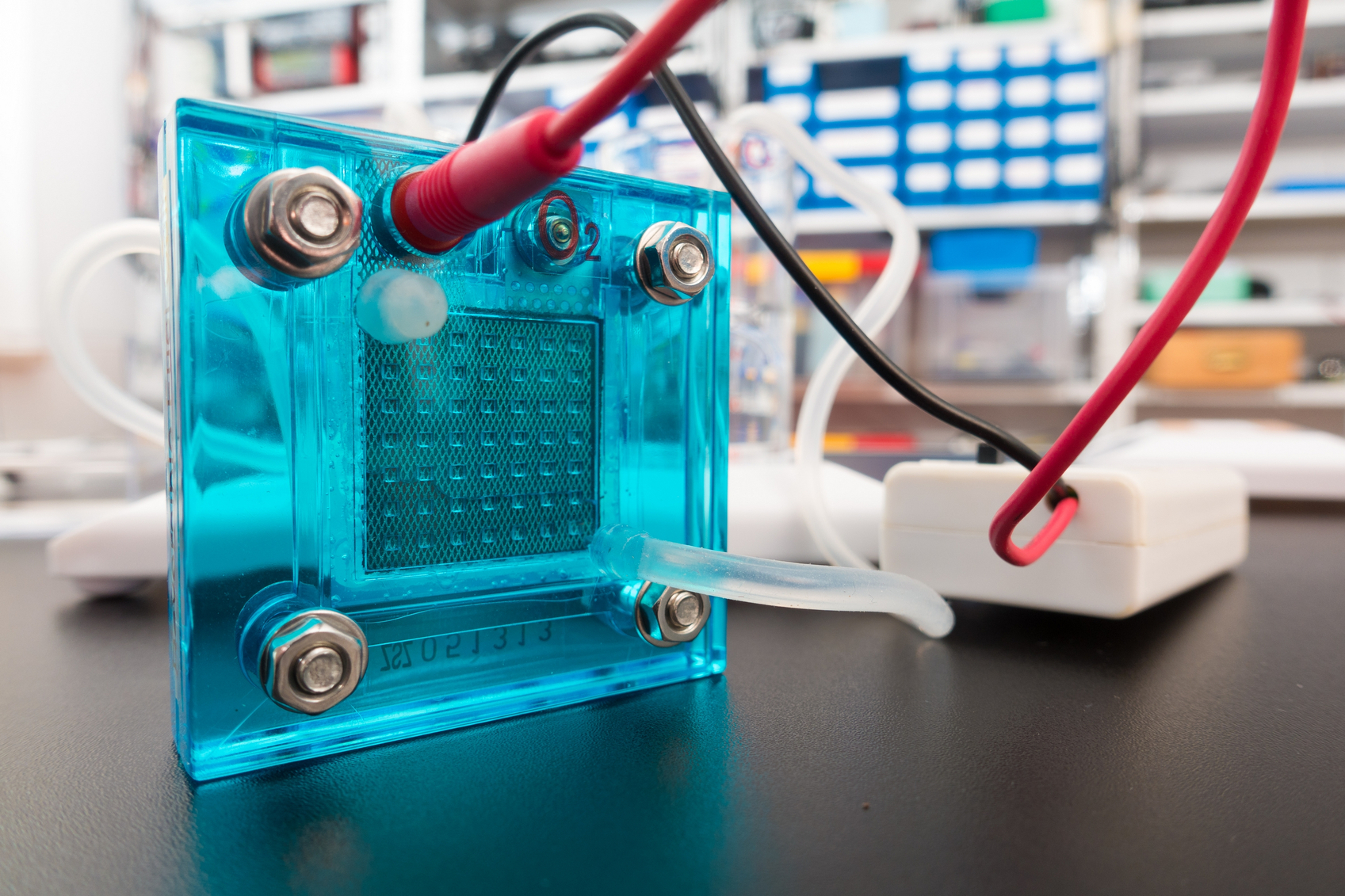 Interest in electric and hybrid vehicles continues to grow across the globe. The
Interest in electric and hybrid vehicles continues to grow across the globe. The 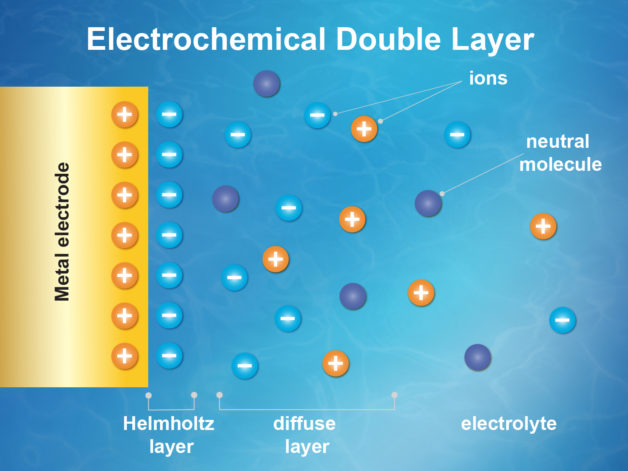
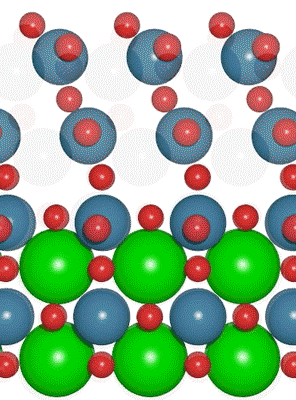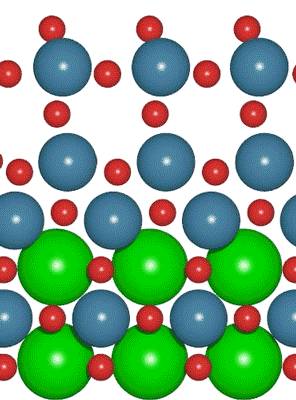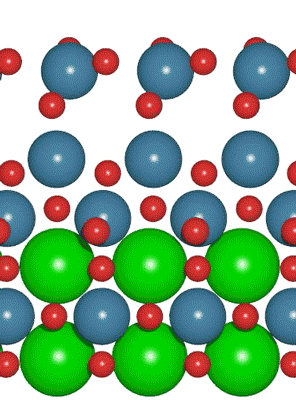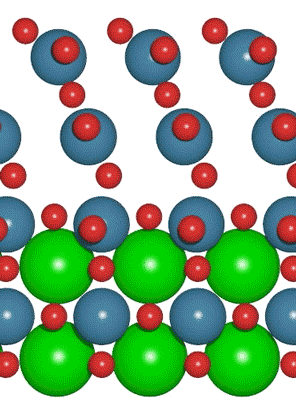 An interdisciplinary team made up of researchers from Stanford University and the U.S. Department of Energy’s SLAC National Accelerator Laboratory recently developed a new catalyst that carries out a solar-powered reaction 100 times faster than ever before.
An interdisciplinary team made up of researchers from Stanford University and the U.S. Department of Energy’s SLAC National Accelerator Laboratory recently developed a new catalyst that carries out a solar-powered reaction 100 times faster than ever before. Deadline: October 1, 2016
Deadline: October 1, 2016 Over the past few years, researchers have been exploring graphene’s amazing properties and vast potential applications. Now, a team from Iowa State University is looking to take those properties enabled by graphene and applied them to sensors and other technologies.
Over the past few years, researchers have been exploring graphene’s amazing properties and vast potential applications. Now, a team from Iowa State University is looking to take those properties enabled by graphene and applied them to sensors and other technologies. With
With  Currently, electric vehicles depend on a complex interplay of batteries and supercapacitors to get you where you’re going. But a recently published paper, co-authored by
Currently, electric vehicles depend on a complex interplay of batteries and supercapacitors to get you where you’re going. But a recently published paper, co-authored by 