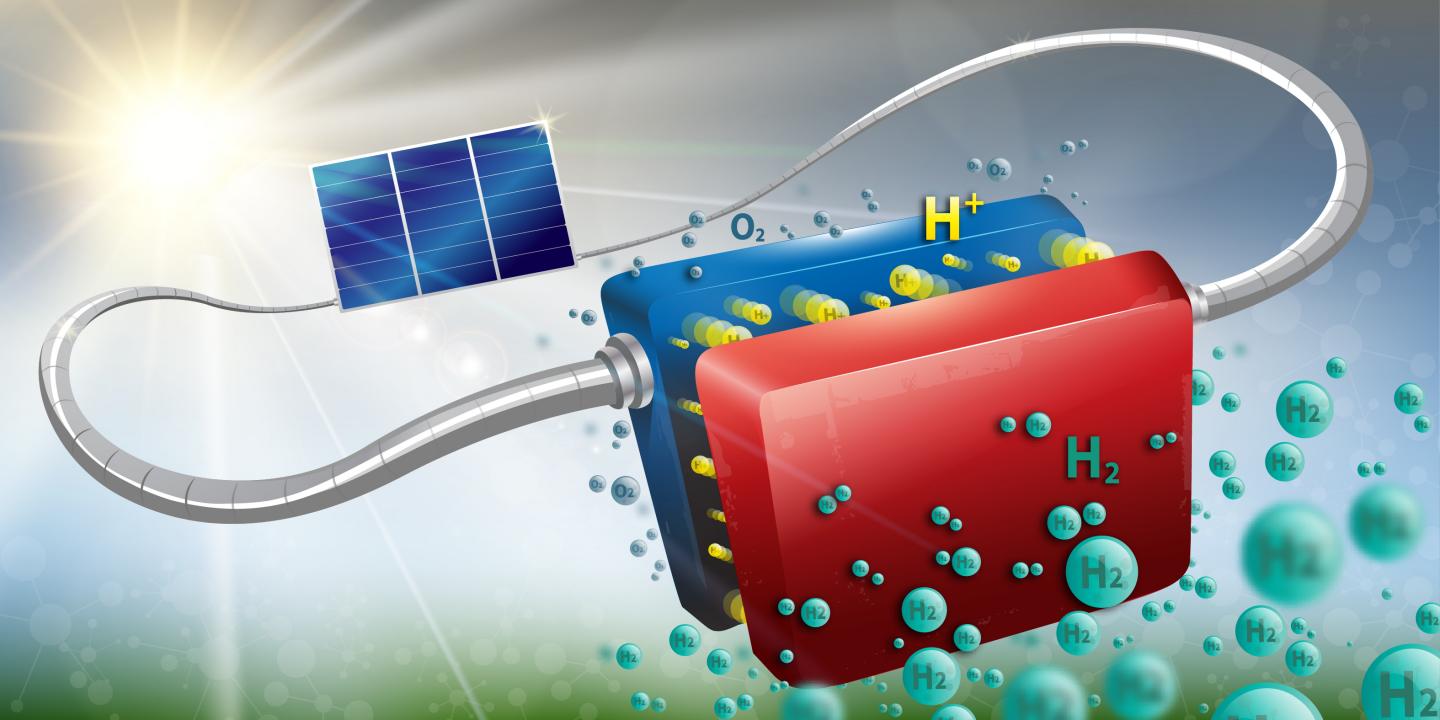
With hydrogen power stations in California, a new Japanese consumer car and portable hydrogen fuel cells for electronics, hydrogen as a zero emission fuel source is now finally becoming a reality for the average consumer. When combined with oxygen in the presence of a catalyst, hydrogen releases energy and bonds with the oxygen to form water.
With hydrogen power stations in California, a new Japanese consumer car and portable hydrogen fuel cells for electronics, hydrogen as a zero emission fuel source is now finally becoming a reality for the average consumer. When combined with oxygen in the presence of a catalyst, hydrogen releases energy and bonds with the oxygen to form water.
The two main difficulties preventing us from having hydrogen power everything we have are storage and production. At the moment, hydrogen production is energy-intensive and expensive. Normally, industrial production of hydrogen requires high temperatures, large facilities and an enormous amount of energy. In fact, it usually comes from fossil fuels like natural gas – and therefore isn’t actually a zero-emission fuel source. Making the process cheaper, efficient and sustainable would go a long way toward making hydrogen a more commonly used fuel.
An excellent – and abundant – source of hydrogen is water. But chemically, that requires reversing the reaction in which hydrogen releases energy when combining with other chemicals. That means we have to put energy into a compound, to get the hydrogen out. Maximizing the efficiency of this process would be significant progress toward a clean-energy future.
One method involves mixing water with a helpful chemical, a catalyst, to reduce the amount of energy needed to break the connections between hydrogen and oxygen atoms. There are several promising catalysts for hydrogen generation, including molybdenum sulfide, graphene and cadmium sulfate. My research focuses on modifying the molecular properties of molybdenum sulfide to make the reaction even more effective and more efficient.
Making hydrogen
Hydrogen is the most abundant element in the universe, but it’s rarely available as pure hydrogen. Rather, it combines with other elements to form a great many chemicals and compounds, such as organic solvents like methanol, and proteins in the human body. Its pure form, H₂, can used as a transportable and efficient fuel.
(more…)
 Using advanced computational methods, University of Wisconsin–Madison materials scientists have discovered new materials that could bring widespread commercial use of solid oxide fuel cells closer to reality.
Using advanced computational methods, University of Wisconsin–Madison materials scientists have discovered new materials that could bring widespread commercial use of solid oxide fuel cells closer to reality.

 Fuel cells play a major role in creating a clean energy future, with a broad set of applications ranging from powering buildings to electrifying transportation. But, as with all emerging technologies, researchers have faced many barriers in developing affordable, efficient fuel cells and creating a way to cleanly produce the hydrogen that powers them.
Fuel cells play a major role in creating a clean energy future, with a broad set of applications ranging from powering buildings to electrifying transportation. But, as with all emerging technologies, researchers have faced many barriers in developing affordable, efficient fuel cells and creating a way to cleanly produce the hydrogen that powers them. Want to see Electrochemistry in Action and ride in one of the world’s first commercial fuel cell cars while at the
Want to see Electrochemistry in Action and ride in one of the world’s first commercial fuel cell cars while at the  Hydrogen has many highly sought after qualities when it comes to clean energy sources. It is a simple element, high in energy, and produces nearly zero harmful emissions. However, while hydrogen is one of the most plentiful elements in the universe, it does not occur naturally as a gas. Instead, we find it combined with other elements, like oxygen in the form of water. For many researchers, water-splitting has been a way to isolate hydrogen for use in cars, houses, and other sustainable fuels.
Hydrogen has many highly sought after qualities when it comes to clean energy sources. It is a simple element, high in energy, and produces nearly zero harmful emissions. However, while hydrogen is one of the most plentiful elements in the universe, it does not occur naturally as a gas. Instead, we find it combined with other elements, like oxygen in the form of water. For many researchers, water-splitting has been a way to isolate hydrogen for use in cars, houses, and other sustainable fuels. Sometimes the biggest advancements are the smallest in size.
Sometimes the biggest advancements are the smallest in size.