A team of researchers from MIT recently demonstrated a new electrochemical method to study thermodynamic processes in an ultra-high temperature molten oxide. In an effort to find new insights into the thermodynamic properties of refractory materials, researchers have developed a container-less electrochemical method to study thermodynamic properties of materials like aluminum oxide, which melts at temperatures above 2,000 degrees Celsius.
The finding were reported in the open access paper, “Electrochemical Study of a Pendant Molten Alumina Droplet and Its Application for Thermodynamic Property Measurements of Al-Ir,” which was recently published in the Journal of The Electrochemical Society.
“We have a new technique which demonstrates that the rules of electrochemistry are followed for these refractory melts,” says senior author Antoine Allanore, an associate professor of metallurgy and member of ECS. “We have now evidence that these melts are very stable at high temperature, they have high conductivity.”


 New research is building a bridge from nature’s chemistry to greener, more efficient synthetic chemistry.
New research is building a bridge from nature’s chemistry to greener, more efficient synthetic chemistry.
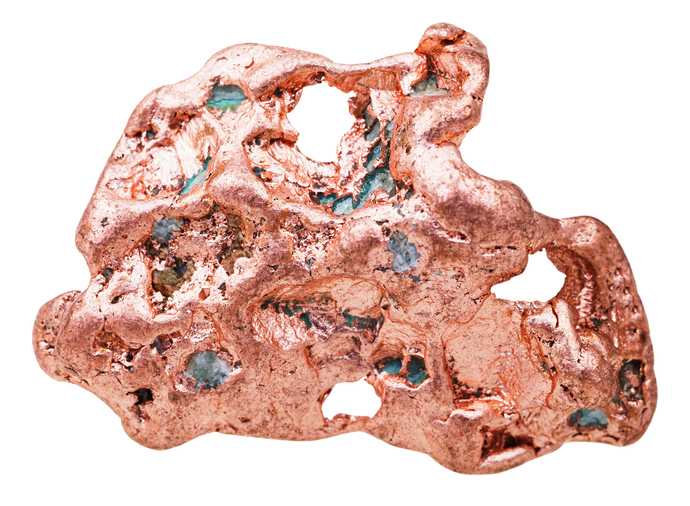 Researchers from MIT have developed a new way to extract copper by separating the commercially valuable metal from sulfide minerals in one step without harmful byproducts. The goal of this new process is to simplify metal production, thereby eliminating harmful byproducts and driving down costs.
Researchers from MIT have developed a new way to extract copper by separating the commercially valuable metal from sulfide minerals in one step without harmful byproducts. The goal of this new process is to simplify metal production, thereby eliminating harmful byproducts and driving down costs.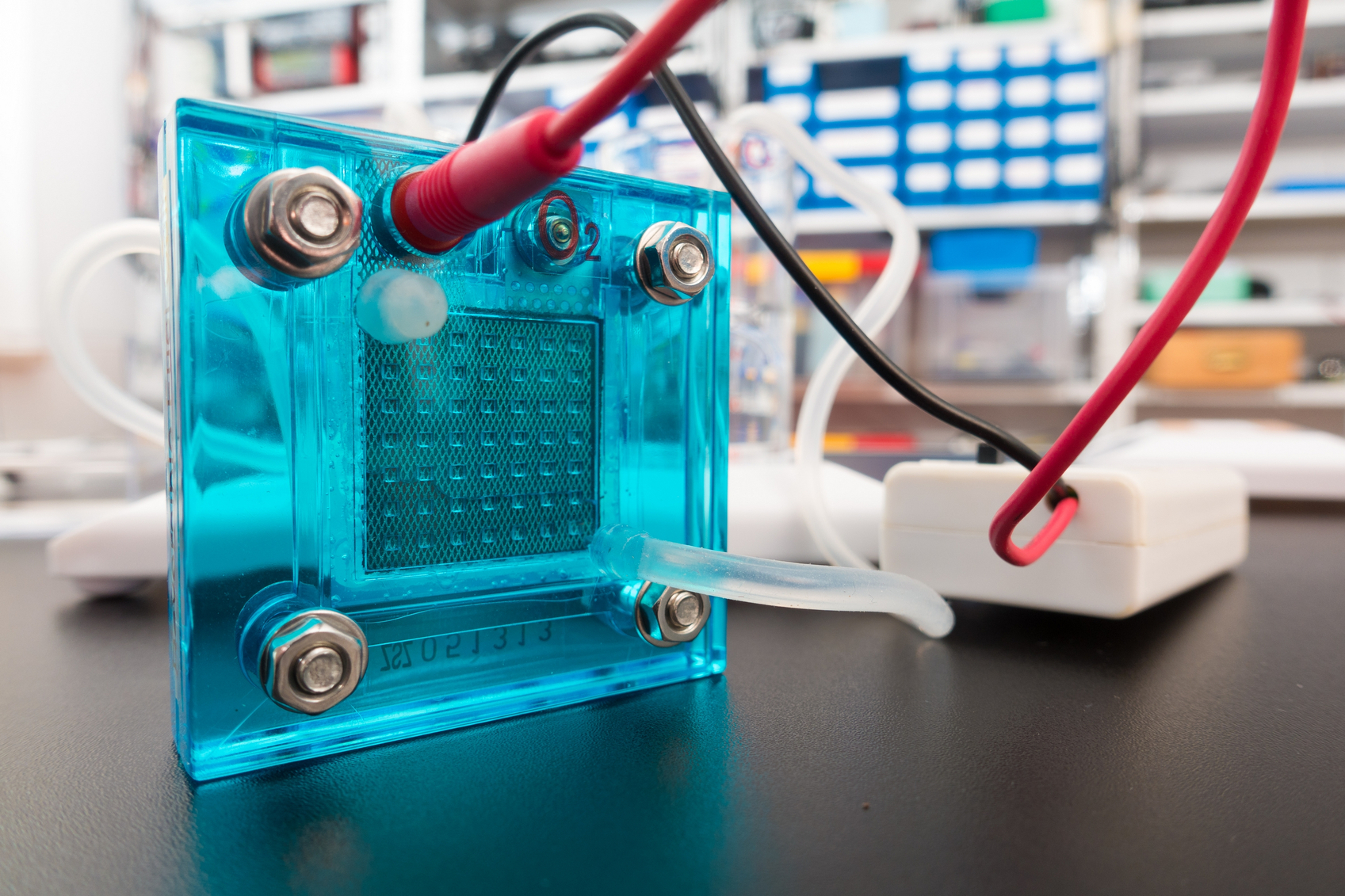 Researchers from Purdue University are making headway on solving issues in electrolyzers and fuel cell development by gaining new insight into electrocatalysts.
Researchers from Purdue University are making headway on solving issues in electrolyzers and fuel cell development by gaining new insight into electrocatalysts. Using high pressure, scientists have created the first high-entropy metal alloy made of common metals to have a hexagonal close-packed (HCP) atomic structure.
Using high pressure, scientists have created the first high-entropy metal alloy made of common metals to have a hexagonal close-packed (HCP) atomic structure. Hydrogen has many highly sought after qualities when it comes to clean energy sources. It is a simple element, high in energy, and produces nearly zero harmful emissions. However, while hydrogen is one of the most plentiful elements in the universe, it does not occur naturally as a gas. Instead, we find it combined with other elements, like oxygen in the form of water. For many researchers, water-splitting has been a way to isolate hydrogen for use in cars, houses, and other sustainable fuels.
Hydrogen has many highly sought after qualities when it comes to clean energy sources. It is a simple element, high in energy, and produces nearly zero harmful emissions. However, while hydrogen is one of the most plentiful elements in the universe, it does not occur naturally as a gas. Instead, we find it combined with other elements, like oxygen in the form of water. For many researchers, water-splitting has been a way to isolate hydrogen for use in cars, houses, and other sustainable fuels. From social to natural and applied sciences, overall scientific output has been growing worldwide – it
From social to natural and applied sciences, overall scientific output has been growing worldwide – it  Wolfram|Alpha
Wolfram|Alpha