 In September 2019, at the 16th International Symposium on Solid Oxide Fuel Cells (SOFC-XVI), Symposium Chair Subhash Singhal presented a plaque from The Electrochemical Society (ECS) to Yukiko Dokiya, the widow of Professor Masayuki Dokiya. Also present were daughter Fumiko Dokiya, her husband Hironobu Dokiya, and their daughter Yoko Dokiya and son Masahiro Dokiya. The plaque thanked the Dokiya family for their generous contribution in Masayuki’s memory. The gift made possible the creation of the Dokiya Fund of The Electrochemical Society in 2004. From 2004 to 2019, the Fund provided financial travel assistance to 128 Dokiya Fund Travel Grant Recipients to attend ECS and other related meetings around the world in their pursuit of electrochemical science and technology to benefit mankind. (more…)
In September 2019, at the 16th International Symposium on Solid Oxide Fuel Cells (SOFC-XVI), Symposium Chair Subhash Singhal presented a plaque from The Electrochemical Society (ECS) to Yukiko Dokiya, the widow of Professor Masayuki Dokiya. Also present were daughter Fumiko Dokiya, her husband Hironobu Dokiya, and their daughter Yoko Dokiya and son Masahiro Dokiya. The plaque thanked the Dokiya family for their generous contribution in Masayuki’s memory. The gift made possible the creation of the Dokiya Fund of The Electrochemical Society in 2004. From 2004 to 2019, the Fund provided financial travel assistance to 128 Dokiya Fund Travel Grant Recipients to attend ECS and other related meetings around the world in their pursuit of electrochemical science and technology to benefit mankind. (more…)
Dahn Unveils Million Mile Battery in Ground-breaking Article
Posted on September 25, 2019 by Frances Chaves Elon Musk promised—and Jeff Dahn delivered! With the publishing of a ground-breaking paper in the Journal of The Electrochemical Society (JES), Dahn announced to the world that Tesla may soon have a battery that makes their robot taxis and long-haul electric trucks viable. Dahn and his research group is Tesla’s battery research partner. Dahn says “… that cells of this type should be able to power an electric vehicle for over one million miles and last at least two decades in grid energy storage.”
Elon Musk promised—and Jeff Dahn delivered! With the publishing of a ground-breaking paper in the Journal of The Electrochemical Society (JES), Dahn announced to the world that Tesla may soon have a battery that makes their robot taxis and long-haul electric trucks viable. Dahn and his research group is Tesla’s battery research partner. Dahn says “… that cells of this type should be able to power an electric vehicle for over one million miles and last at least two decades in grid energy storage.”
According to Doron Aurbach, JES batteries and energy storage technical editor, “This comprehensive article is expected to be impactful in the field of batteries and energy storage. It is a very systematic study by one of the most renowned and prestigious electrochemistry groups in the world. It was a pleasure for me as a technical editor to handle this paper. It substantiates all the statements about the truly high quality and importance of JES, one of the leading and most prestigious journals in electrochemistry. JES provides an excellent service to the global electrochemistry community—and thousands of ECS members—regardless of ‘impact factors.’” As of today, Dahn’s JES article has received over 31,563 abstract views, over 17,000 articles downloads, and quotes in news outlets around the world. (more…)
Call for Nominations: JES Technical Editor in Fuel Cells, Electrolyzers, and Energy Conversion
Posted on July 26, 2019 by Andrew Ryan Deadline: September 13, 2019
Deadline: September 13, 2019
ECS is seeking to fill the position of technical editor in fuel cells, electrolyzers, and energy conversion for the Journal of The Electrochemical Society (JES).
Nominees for this position must possess and maintain scientific knowledge of the scope of the fuel cells, electrolyzers, and energy conversion topical interest area, which covers theoretical and experimental aspects of all types of fuel cells, electrolyzers, photovoltaics, and photoelectrochemistry. Specific topics as relates to energy conversion include design, modeling, testing, and evaluation; novel electrode structures and their characterization, including electrocatalytic materials and electrocatalysis; engineering aspects of fuel, electrochemical fuel synthesis, water, and thermal management. Materials at high temperatures are included.
The technical editor will be appointed for a minimum initial two-year term, renewable for additional terms, up to a maximum of 12 years total service in this role.
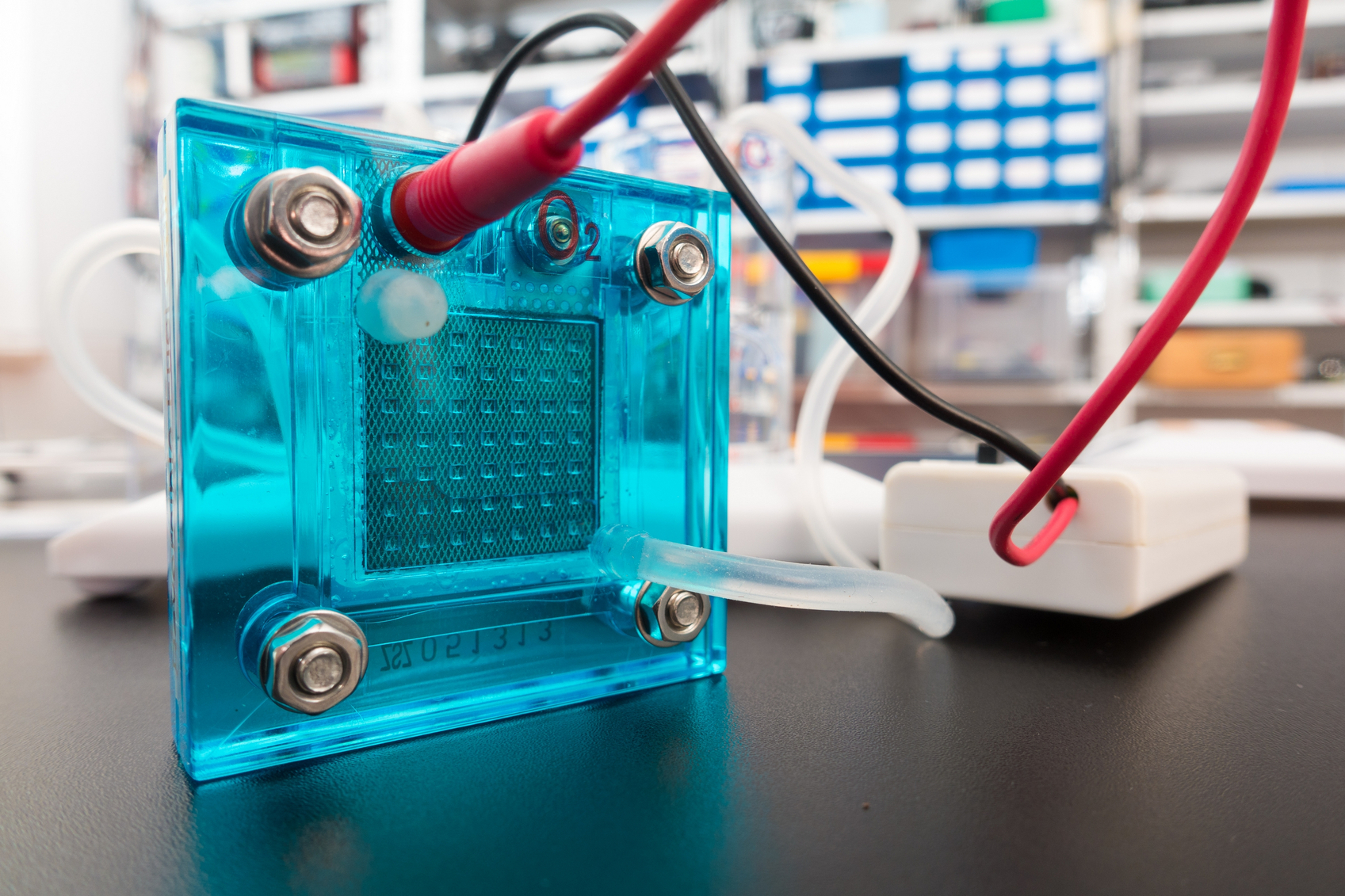
 Fuel cells play a major role in creating a clean energy future, with a broad set of applications ranging from powering buildings to electrifying transportation. But, as with all emerging technologies, researchers have faced many barriers in developing affordable, efficient fuel cells and creating a way to cleanly produce the hydrogen that powers them.
Fuel cells play a major role in creating a clean energy future, with a broad set of applications ranging from powering buildings to electrifying transportation. But, as with all emerging technologies, researchers have faced many barriers in developing affordable, efficient fuel cells and creating a way to cleanly produce the hydrogen that powers them.
In a new Perspective article, published in the Journal of The Electrochemical Society, researchers are aiming to tackle a fundamental debate in key reactions behind fuel cells and hydrogen production, which, if solved, could significantly bolster clean energy technologies.
In the open access article, “Perspective—Towards Establishing Apparent Hydrogen Binding Energy as the Descriptor for Hydrogen Oxidation/Evolution Reactions,” Yushan Yan and his coauthors from the University of Delaware provide an authoritative overview of work done in the areas of hydrogen oxidation and evolution, present key questions for debate, and provide paths for future innovation in the field.
 Nitrogen-doped carbon nanotubes or modified graphene nanoribbons could be effective, less costly replacements for expensive platinum in fuel cells, according to a new study.
Nitrogen-doped carbon nanotubes or modified graphene nanoribbons could be effective, less costly replacements for expensive platinum in fuel cells, according to a new study.
In fuel cells, platinum is used for fast oxygen reduction, the key reaction that transforms chemical energy into electricity.
The findings come from computer simulations scientists created to see how carbon nanomaterials could be improved for fuel-cell cathodes. Their study reveals the atom-level mechanisms by which doped nanomaterials catalyze oxygen reduction reactions (ORR).
Doping with nitrogen
Boris Yakobson, a professor of materials science and nanoengineering and of chemistry at Rice University, and his colleagues are among many researchers looking for a way to speed up ORR for fuel cells, which were discovered in the 19th century but not widely used until the latter part of the 20th. Fuel cells have since powered transportation modes ranging from cars and buses to spacecraft.
 Applying a tiny coating of costly platinum just 1 nanometer thick—about 1/100,000th the width of a human hair—to a core of much cheaper cobalt could bring down the cost of fuel cells.
Applying a tiny coating of costly platinum just 1 nanometer thick—about 1/100,000th the width of a human hair—to a core of much cheaper cobalt could bring down the cost of fuel cells.
This microscopic marriage could become a crucial catalyst in new fuel cells that use generate electricity from hydrogen fuel to power cars and other machines. The new fuel cell design would require far less platinum, a very rare metal that sold for almost $900 an ounce the day this article was produced.
“This technique could accelerate our launch out of the fossil-fuel era,” says Chao Wang, an assistant professor of chemical and biomolecular engineering at Johns Hopkins University and senior author of a study published in the journal Nano Letters.
“It will not only reduce the cost of fuel cells,” Wang says. “It will also improve the energy efficiency and power performance of clean electric vehicles powered by hydrogen.”
‘Tiny Windows’ Test May Find Better 2D Materials for Fuel Cells
Posted on October 9, 2017 by Amanda Staller A closer look at catalysts is giving researchers a better sense of how these atom-thick materials produce hydrogen.
A closer look at catalysts is giving researchers a better sense of how these atom-thick materials produce hydrogen.
Their findings could accelerate the development of 2D materials for energy applications, such as fuel cells.
The researchers’ technique allows them to probe through tiny “windows” created by an electron beam and measure the catalytic activity of molybdenum disulfide, a two-dimensional material that shows promise for applications that use electrocatalysis to extract hydrogen from water.
Initial tests on two variations of the material proved that most production is coming from the thin sheets’ edges.
Researchers already knew the edges of 2D materials are where the catalytic action is, so any information that helps maximize it is valuable, says Jun Lou, a professor of materials science and nanoengineering at Rice University whose lab developed the technique with colleagues at Los Alamos National Laboratory.
Our guest today, James Fenton, is the director of the Florida Solar Energy Center at the University of Central Florida – the nation’s largest and most active state-supported renewable energy and energy efficiency institute.
Fenton is also the current secretary of the ECS Board of Directors.
Listen to the podcast and download this episode and others for free through the iTunes Store, SoundCloud, or our RSS Feed. You can also find us on Stitcher.
Image: CC0 Public Domain
Researchers have created a way to look inside fuel cells to see the chemical processes that lead them to breakdown.
Fuel cells could someday generate electricity for nearly any device that’s battery-powered, including automobiles, laptops, and cellphones. Typically using hydrogen as fuel and air as an oxidant, fuel cells are cleaner than internal combustion engines because they produce power via electrochemical reactions. Since water is their primary product, they considerably reduce pollution.
The oxidation, or breakdown, of a fuel cell’s central electrolyte membrane can shorten their lifespan. The process leads to formation of holes in the membrane and can ultimately cause a chemical short circuit. Engineers created the new technique to examine the rate at which this oxidation occurs with hopes of finding out how to make fuel cells last longer.
Using fluorescence spectroscopy inside the fuel cell, they are able to probe the formation of the chemicals responsible for the oxidation, namely free radicals, during operation. The technique could be a game changer when it comes to understanding how the cells break down, and designing mitigation strategies that would extend the fuel cell’s lifetime.
“If you buy a device—a car, a cell phone—you want it to last as long as possible,” says Vijay Ramani, professor of environment & energy at the School of Engineering & Applied Science at Washington University in St. Louis.

Image: CC0 Public Domain
Researchers at Los Alamos National Laboratory (LANL) are taking a closer look at fuel cell catalysts in hopes of finding a viable alternative to the expensive platinum and platinum-group metal catalysts currently used in fuel cell electrodes. Developments in this area could lead to more affordable next-generation polymer electrolyte fuel cells for vehicles.
The research, led by ECS fellow Piotr Zelenay, looks at the fuel cell catalysts at the atomic level, providing unique insight into the efficiency of non-precious metals for automotive and other applications.
“What makes this exploration especially important is that it enhances our understanding of exactly why these alternative catalysts are active,” Zelenay says. “We’ve been advancing the field, but without understanding the sources of activity; without the structural and functional insights, further progress was going to be very difficult.”
This from LANL:
Platinum aids in both the electrocatalytic oxidation of hydrogen fuel at the anode and electrocatalytic reduction of oxygen from air at the cathode, producing usable electricity. Finding a viable, low-cost PGM-free catalyst alternative is becoming more and more possible, but understanding exactly where and how catalysis is occurring in these new materials has been a long-standing challenge. This is true, Zelenay noted, especially in the fuel cell cathode, where a relatively slow oxygen reduction reaction, or ORR, takes place that requires significant ‘loading’ of platinum.

