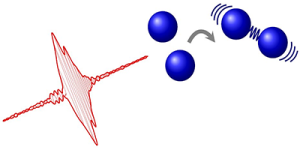
Tailored laser pulse controls the formation of a molecular bond between two atoms.
Image: Christiane Koch
Until now, the idea of controlling reactions with the light from lasers was only theoretical. However, new research shows that a laser pulse has the ability to control the formation of a molecular bond between two atoms.
Due to this new development, researchers can now control the path of the chemical process with extreme precision.
This from APS Physics:
For the first time, researchers demonstrate the coherent control of the reaction by which two atoms form a molecule. The achievement—coupled with other photocatalyst tools—could potentially lead to a chemical assembly line, in which lasers slice and weld molecular pieces into a desired end product.
The new development resulted from the collaboration between experimentalists from Technion-Israel Institute of Technology and theorists from Germany’s University of Kassel. The team recently published their finding in the journal Physical Review Letters.
“Coherent control, that is the use of matter–wave interferences [in atoms] to steer a dynamical process towards a certain target [using light], has always fascinated me,” says Christiane Koch from the University of Kassel, who carried out the work. “It seemed very unsatisfactory that even though [it] was conceived to steer chemical reactions, nobody seemed to be able to do it.”
To achieve the desired results, the researchers shot short pulses of light at a group of magnesium atoms. By adjusting the pulse shape, the team saw that they could increase the number of molecules formed.
“We have had difficulties in getting this research funded because the reviewers did not believe that the process could work,” Koch tells Chemistry World. “We hope that these accomplishments will motivate other groups to look again into the coherent control of chemical reactions and thus revive the field.”
Head over to the Digital Library to read more about chemical bonds.

