The Electrochemical Society hosted Dr. Judy Jeevarajan’s live webinar, “Characterization of Li-ion Battery Thermal Runaway in ESSs and EVs,” on August 3, 2022. Dr. Jeevarajan took audience questions during a live Question and Answer session at the end of the presentation. She kindly answered, in writing, questions not answered during the broadcast. Find these responses below.
The Electrochemical Society hosted Dr. Judy Jeevarajan’s live webinar, “Characterization of Li-ion Battery Thermal Runaway in ESSs and EVs,” on August 3, 2022. Dr. Jeevarajan took audience questions during a live Question and Answer session at the end of the presentation. She kindly answered, in writing, questions not answered during the broadcast. Find these responses below.
NOTE: Registration is required to view the webinar.
Q&A
Did you consider IR scanning instead of physical T/C to measure the temps?
Yes, we considered it. For a majority of our tests, IR thermal imaging was not possible due to the chamber we were using. Also, we did not want to place our IR camera inside the test chamber, as there was always the threat of the camera being destroyed during the thermal runaway.
In conducting the thermal runaway tests, were the cells loaded to draw the designed Ah or Wh values? Also, was the test done at elevated temperatures while being electrically loaded? Could electrical stress contribute to the initiation of thermal runaway?
No, cells did not have any loads since we were working on designs for transportation (where cells and batteries do not power anything). Electrical stress can contribute to the initiation if the charge or discharge protocols were beyond what the cells or battery could handle.
Does the UL offer a hands-on training/workshop on this topic?
Not at this time.
What was the Cell suppliers limit for storage temperatures?
The specification sheets do not provide storage temperatures, only max environmental temperatures for charge and discharge.
Is the max pressure of a pouch cell lower or higher than cylindrical cells and prismatic cells during thermal runaway? What is the max pressure of pouch cells during thermal runaway?
Pouch cells open up as low as 50 psi, while typical 18650 model cylindrical cells vent at greater than 700 psi. The pressure released by each during a thermal runaway depends greatly on the distance at which it is measured. For cylindrical 18650 type cells, the max pressure we have measured during thermal runaway is 4 psi.
What are the best parameters to monitor in order to detect Thermal Runaway Event the fastest? For example, concentration of specific gases, pressure (in a sealed or semi-sealed battery compartment), air temp, packaging temp etc.?
The best parameters are voltage and temperature. Voltages allow one to determine when the initial vent and final thermal runaway occur. Temperature measurements, if placed appropriately, track the same very well.
What is the composition of the NMC cells used?
We do not have details of the ratios of metals in the NMC.
Can the Nail penetration test help us to prevent thermal runaway?
Nail penetration tests cannot help prevent thermal runaway. But, if done correctly, they may help to understand the propensity of a certain cell design at the certain SOC and temperature to go into thermal runaway.
Among all tests, what is the single most significant thermal propagation mode (gas/spills, conduction, etc.)?
Heat transfer through convection and conduction and somewhat due to radiation (depends on cell design and mitigation materials), gas and electrolyte leaks can also add to propagation. But heat needs to be present for electrolyte liquids or vapors to become a source of thermal runaway.
Would mathematical modeling be relevant to predict runaway events (and of course thermal propagation), given high dispersion of results?
Yes, if the models take into account high-fidelity data.
Is there an established standard method for elucidating thermal runaway in batteries?
Most standards call out the use of the heating tape.
What are your thoughts on batteries containing “nonflammable” electrolytes? Are these relatively safe from thermal runaway? Do they require any modifications to the thermal runaway tests presented?
Cells with non-flammable electrolytes should be tested to confirm that they do provide the property. Cycle life aging should also be performed to confirm that the non-flammable electrolyte provides it properties after aging. Cells with non-flammable electrolyte do not require any modifications before test.
How can we mitigate the fire issues of NMC based Li-ion batteries?
Understanding the thermal design in the batteries, the thermal gradient, and the worst case thermal locations will help in designing mitigation measures, either with mitigation materials or container design to remove heat faster than it is created in the batteries. This can reduce the risk of fires in any Li-ion battery.
Is a BMS with control systems able to inform about battery safety issues in EVs?
A well-designed BMS should be able to inform on battery safety issues in EVs.
How does Li dendrite formation damage the separators in a Li-ion battery?
Li dendrites can be formed due to low temperature charging, or due to high rates of charging, or charging beyond the manufacturer-recommended voltage limit. If the dendrites grow perpendicular to the electrode surface, then they can penetrate the separator, cause temperature to rise, and damage the separator.
Where do you recommend getting the best advice for firefighters dealing with ESS fires involving Li-ion batteries?
Fire suppression is still being optimized. There are a few studies in the literature that discuss the use of various fire suppressants. Fire experts should be contacted for such details. We are open to discussions on this subject since we have been performing some research in this area.
Very advanced flexible electronic devices are developed. So, is it possible to make flexible Li-ion cells to sustain a long life without much heat or explosions?
Yes. There are some thin flexible designs that are available today. The key is to confirm that there is no break in the separator during the flexing process so the electrodes do not touch each other and experience a short circuit.
Do you have any gas release information for HF (hydrogen fluoride)?
We do know that HF is released and there is at least one study from Chalmers University of Technology, Sweden, that discusses the production of HF. It is difficult to get a good detection of HF due to its reaction with chamber materials and due to its condensation on the walls of the chamber. We are still working on optimizing the detection of HF.
Why is 2mm thickness optimal for preventing propagation?
We have done a lot of work in this area (see papers published in 2015) with various thicknesses. We found that anything more than 2 mm might work but that would cause other issues like large battery containers and also more IR drop in a battery design.
Why was the temperature of a single cell higher above the heating element than below? How did you control heat dissipation?
The heat distribution on the cell is pretty uniform and it flows in the axial direction for the Li-ion cylindrical cells. If there was a bigger difference, it is probably because of heat dissipation from that part of the cell that caused it to be lower than the top part of the cell. So, how the cell is held can make a difference.
Since pouch cell is subjected to expansion during charging, how could the influence of the foam and no foam scenario impact the thermal runaway severity?
Not sure what this question is referring to. Depending on the type of foam, it may or may not allow the required expansion of the pouch cell. In general, prismatic cell designs (pouch or metal can) required restraints on the flat face of the cells to prevent expansion. The pressures required for the restraints are determined by the manufacturer.
Could the use of metal-coated polymer electrodes in commercial batteries reduce the thermal runaway?
I have not worked with metal-coated polymer electrodes.
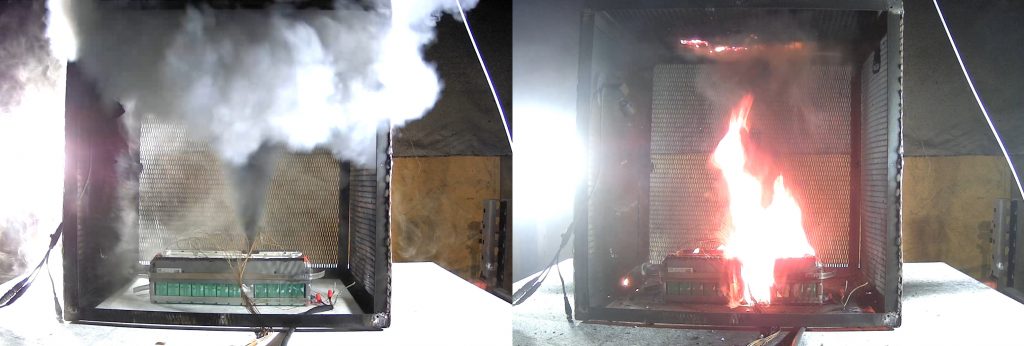
A cell at a 3 percent SOC has less active material, so how could the thermal runaway be more severe?
I mentioned that this was an anomaly. The cells were manufactured with low quality. Hence, the cells vented liquid electrolyte when subjected to the thermal runaway test. Also, because the liquid electrolyte has components that have a low flash point, it is possible that the vapors combusted and as the vapors spread and touched the hot surfaces of the neighboring cells, they underwent propagation, too. The open box tests were carried out to record this event and understand it.
Should the thermal runaway experiments be carried out in inert atmospheres?
It depends on the intent of the test. If you would like to determine the products of a thermal runaway and a fire, then you do not use inert atmospheres. If you want to know the products of thermal runaway without combustion, then you carry out the tests in an inert atmosphere.
For a high C rate pouch cell, what is the best characterization model to use? HPPC test, EIS or power discharge? ECM and NTGK model?
The load depends on the application.
Which negative electrodes were used in the test cells and electrolyte?
The negative electrodes are graphite. We do not have the composition of the electrolyte. It is a commercial cell.
Have you characterized the cell components after the thermal runaway and what are the results?
Depending on the nature of the thermal runaway, there may not be anything left to analyze.
Why do many Korean ESS often fire? Is the root cause cleared?
The news article indicated 23 fires. I do not know if the root cause was cleared. I only know what the news articles mention.
Could you provide more details about how you performed the gas analysis part of the experiment?
There were two types of tests carried out. One was in an open environment using the cone calorimeter method. The other was in the closed chamber using the ASTM E918 method.
Did you consider the manufacturer battery thickness of the electrode materials used for all tests?
The cells are commercial cells and have been used as a test vehicle for many years.
Have you done any atomistic computational study for better predictions to compare with your lab work?
We have started doing that with the new modeling experts in our team.
What kind of extinguisher or medium should we use to deal with a thermal runaway situation or lithium ion battery fire (both NMC and LFP)?
We found water to be successful so far. Other commercial extinguishers have also been shown to be effective.
In terms of a single cell, in your opinion, what type is the safest (cylindrical or pouch)?
One can take a safe cell and make an unsafe battery or vice versa. So battery design, stringent testing in the relevant configuration and environment, and stringent screening of cells and batteries are the critical factors in determining the safety of a battery design.
Have you used any software to model reaction (including combustion) and heat transfer in batteries, to gain more insight into mechanisms and important parameters?
Yes. Our modeling experts use at least three different software platforms to model thermal runaway.
Were these thermal runaway propagation studies repeatable? That is, if you would run the same experiments, under same conditions, was the propagation behavior the same as seen before?
Yes, at least three trials were performed under each condition, sometimes more. Our results are very consistent.
If there is notable variability in thermal runaway within the same type of battery, which would get amplified for a large pack, how would you recommend one design suitable mitigation strategies? Do we model it based on just one experiment, or treat it as a statistical problem, with repeated experiments?
Results should be repeatable and consistent for at least three tests. If variability is observed, one needs to understand the cause of the variability. If that is the inherent property, then one needs to design for worst case results observed.
What is the significance of the different manufacturing location resulting in that anomaly? Are there less controls in that location which cause the cells to be more susceptible to thermal runaway?
It is possible that the quality of the cell manufacturing process was not the best. We did not observe thermal runaway with the 3 percent SOC cells in the batches we received after that, when we were specific about the manufacturing location.
Where does CO/CO2 gas come from? Why do LFP and NMC have different CO levels at high temp?
CO comes from the breakdown of the carbonates in the electrolyte. CO2 is formed when CO is oxidized by oxygen from the cathode breakdown or from air. The reason the LFP cell had a higher level of CO2 and no CO in our results was because the cells had undergone complete combustion and charring that resulted in all the CO being oxidized to CO2. The NMC cell did not undergo similar fires and did not show any external charring.