 Kyle C. Smith
Kyle C. Smith
Associate Professor, Mechanical Science and Engineering
University of Illinois Urbana-Champaign
Date: May 7, 2025
Time: 1300-1400h ET
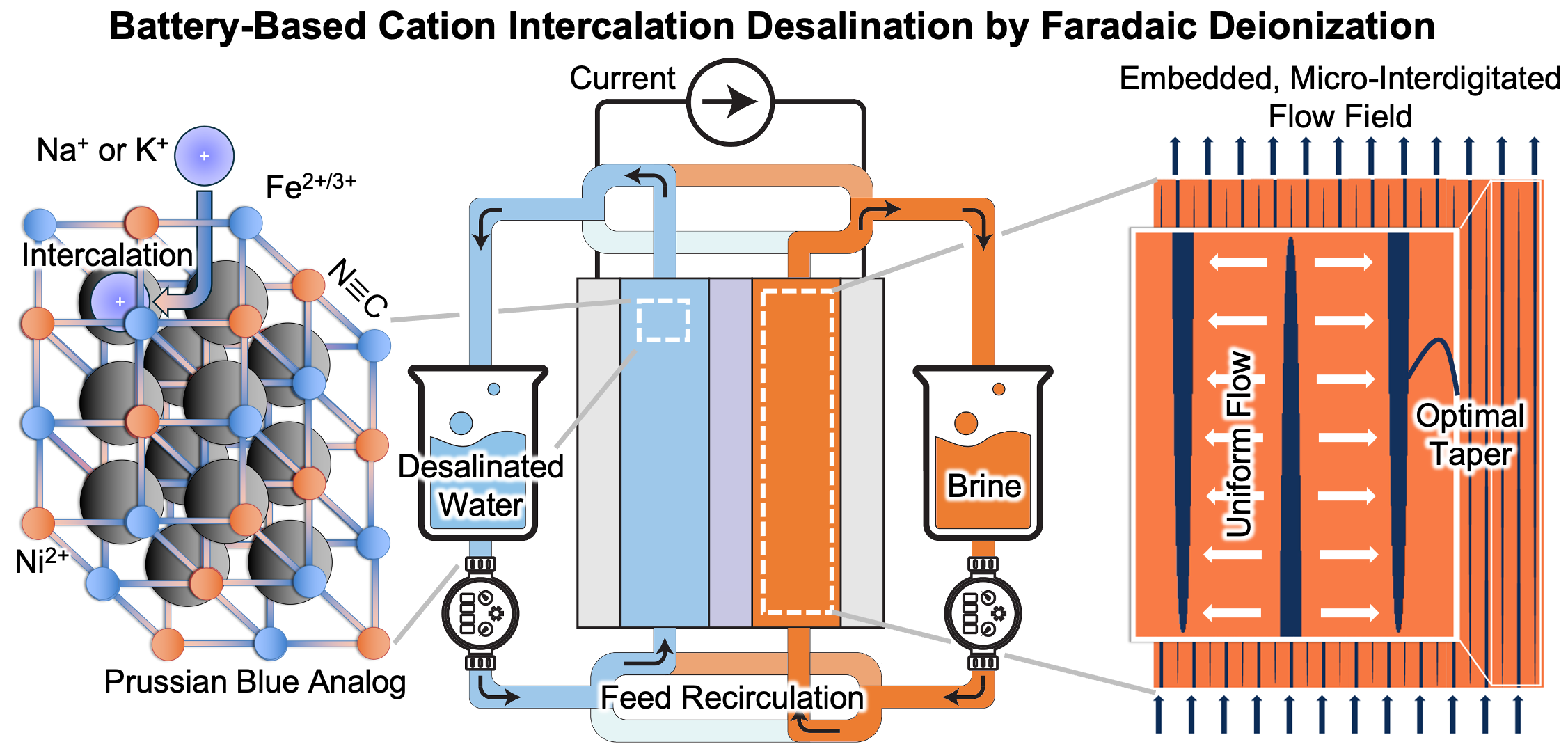
With increased water scarcity and global warming looming, electrochemical technology offers low-energy mitigation pathways via desalination and carbon capture. This webinar demonstrates how the >5 molar solid-state concentration swings afforded by cation intercalation materials—used originally in rocking-chair batteries—can effect desalination using Faradaic deionization (FDI). We show how the salt depletion/accumulation effect—that plagues Li-ion battery capacity under fast charging conditions—is exploited in a symmetric Na-ion battery to achieve seawater desalination, exceeding by an order of magnitude the limits of capacitive deionization with electric double layers. While initial modeling that introduced such an architecture blazed the trail for the development of new and old intercalation materials in FDI, experimental demonstration of seawater-level desalination using Prussian blue analogs required cell engineering to overcome the performance-degrading processes that are unique to the cycling of intercalation electrodes in the presence of flow. This led to innovative embedded, micro-interdigitated flow fields with broader application toward fuel cells, flow batteries, and other flow-based electrochemical devices. Similar symmetric FDI architectures using proton intercalation materials are also shown to facilitate direct-air capture of CO2 with unprecedentedly low energy input by reversibly shifting pH within aqueous electrolyte.
Benefits of attending the webinar
- Learn how cation intercalation electrodes can be used for desalination
- Develop understanding of flow and reactions in interdigitated flow fields embedded in intercalation electrodes
- Learn how proton intercalation electrodes can be used for CO2 capture
Presenter
Kyle C. Smith is Associate Professor in the Mechanical Science and Engineering at the University of Illinois Urbana-Champaign (UIUC). His group uses understanding of flow, transport, and thermodynamics in electrochemical devices and materials to innovate toward separations, energy storage, and conversion.
Prof. Smith joined the UIUC faculty in 2014 after completing his PhD in Mechanical Engineering at Purdue University (2012) and post-doc in materials science and engineering at the Massachusetts Institute of Technology (2014). He received the 2024 UIUC Grainger College Dean’s Award for Early Innovation as an Associate Professor and 2018 International Society of Electrochemistry Elsevier Prize in Applied Electrochemistry. Among his 59 journal papers and 14 patents and patents pending, his Journal of the Electrochemical Society article introducing Na-ion battery-based desalination using porous electrode theory [Smith and Dmello, J. Electrochem. Soc., 163, p. A530 (2016)] was among the top ten most downloaded for five months in 2016. His group was also the first to experimentally demonstrate seawater-level salt removal using this approach [Do et al., Energy Environ. Sci., 16, p. 3025 (2023); Rahman et al., Electrochimica Acta, 514, p. 145632 (2025)], introducing flow fields embedded in electrodes to do so.
Learn more about upcoming ECS Webinars and review our previous webinar recordings.
Interested in presenting in the ECS Webinar Series?
Email your presentation title and abstract to education@electrochem.org for consideration.