 Interest in electric and hybrid vehicles continues to grow across the globe. The world economy saw EV sales go from around 315,000 in 2014 to 536,000 in 2015, and trends so far for 2016 show that the number of vehicles sold this year is on track to far exceed numbers we’ve seen in previous years.
Interest in electric and hybrid vehicles continues to grow across the globe. The world economy saw EV sales go from around 315,000 in 2014 to 536,000 in 2015, and trends so far for 2016 show that the number of vehicles sold this year is on track to far exceed numbers we’ve seen in previous years.
Moving EVs forward
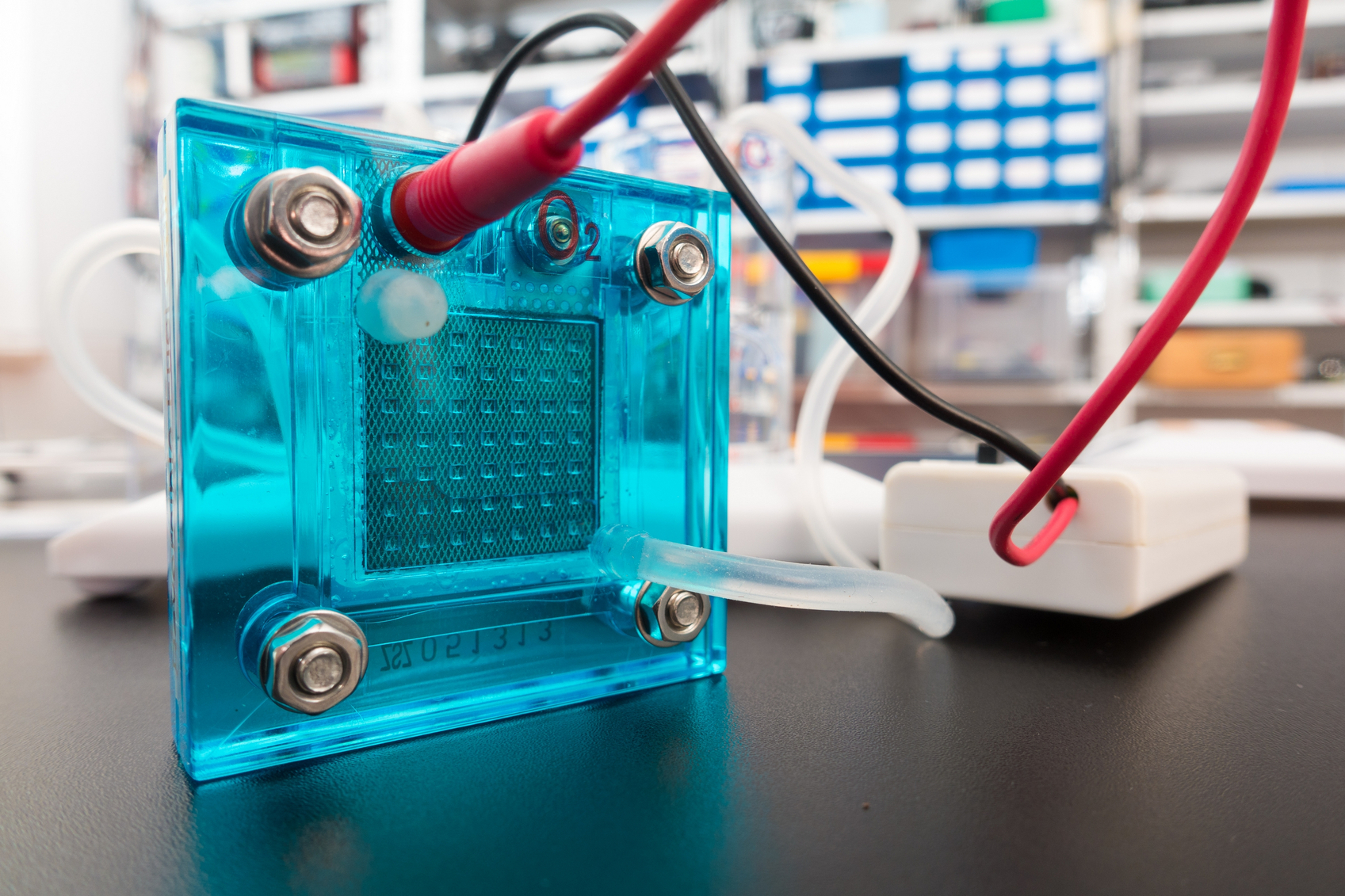
But in order to make these cars, there needs to be an energy storage source that is not only sustainable, but cheap to produce, with high efficiency, and can be easily mass produced. One of the leading contenders in that race has become fuel cell technology.
In recent years, new materials and better heat management processes have advanced fuel cells. Now, researchers from Lawrence Berkeley National Lab’s NERSC center (including ECS Fellow Radoslav Adzic and ECS member Kotaro Sasaki) are putting their chips on polymer electrolyte fuel cells (PEFCs) to be at the forefront of fuel cell technology due recent finds. In a new study, the group showed that PEFCs could be made to run more efficiently and produced more cost-effectively by reducing the amount of a single key ingredient: platinum.
Laboratory curiosity
While fuel cells date back to 1839, they spent a majority of their existence as laboratory curiosities. It wasn’t until the 1950s when fuel cells finally made their way to the main stage, eventually going on to power the Gemini and Apollo space flights in the 1960s.
Now, fuel cells have become a serious contender in energy storage and are taking steps forward to become more efficient and cost-effective.
“Even though PEFCs are well known as an ideal electrical power system, the utilization of platinum hinders the cost-effective commercialization of these type of fuel cells,” says Yongman Choi, co-author of the study.
This from NERSC:
While some non-precious metal-based catalysts have demonstrated enhanced activity, their oxygen reduction reaction (ORR) activity is still inferior to that of platinum-based catalysts, resulting in a much thicker electrode. Thus Choi and his colleagues DFT calculations to test a non-precious-metal-based cathode material containing only a small amount of platinum. Their calculations, which used approximately 300,000 core hours at NERSC—a DOE Office of Science user facility—confirmed that platinum nanoclusters on metal–nitrogen doped ordered mesoporous porphyrinic carbon significantly enhance the ORR activity.

