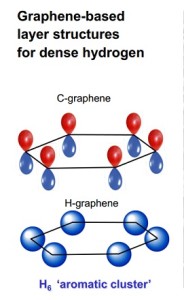
A comparison of the basic ring structure of the carbon compound graphene with that of a similar hydrogen-based structure synthesized by Carnegie scientists.
Credit: Carnegie Science
A new study shows remarkable parallels between hydrogen and graphene under extreme pressures.
The study was conducted by Carnegie’s Ivan Naumov and Russell Hemley, and can be found in the December issue of Accounts of Chemical Research.
Because of hydrogen’s simplicity and abundance, it has long been used as a testing ground for theories of the chemical bond. It is necessary to understand chemical bonding in extreme environments in order to expand our knowledge of a broad range of conditions found in the universe.
It has always been difficult for researchers to observe hydrogen’s behavior under very high pressure, until recently when teams observed the element at pressures of 2-to-3.5 million times the normal atmospheric pressure.
Under this pressure, it transforms into an unexpected structure that consists of layered sheets, rather than close-packed metal – which had been the prediction of scientists many years ago.
This from Carnegie Science:
These hydrogen sheets resemble the carbon compound graphene. Graphene’s layers are each constructed of a honeycomb structure made of six-atom carbon rings. This conventional carbon graphene, first synthesized about a decade ago, is very light, but incredibly strong, and conducts heat and electricity very efficiently. These properties promise revolutionary technology, including advanced optical electronics for screens, high-functioning photovoltaic cells, and enhanced batteries and other energy storage devices.
The study also contradicts theoretical calculations going back to the 1930’s, where scientists believed that hydrogen initially becomes a shiny metal and a good conductor. Instead, the initial stages of hydrogen produce a dark, poorly conducting metal like graphite.
“Overall, our results indicate that chemical bonding occurs over a much broader range of conditions than people had previously considered. However, the structural effects of that chemical bonding under extreme conditions can be very different than that observed under the ordinary conditions that are familiar to us,” Hemley said.
Just like Naumov and Hemley, the scientists at ECS are also looking for new qualities and innovation in hydrogen. Head over to the Digital Library and check out the latest research!
And make sure to get our RSS Feed so you can track updates as soon as they become available.

