 The Electrochemical Society hosted Kyle C. Smith’s live webinar, “Intercalation-Based Desalination and Carbon Capture for Water and Climate Sustainability” on May 7, 2025. A live Question and Answer (Q&A) session followed. Dr. Smith’s written answers to questions not addressed during the broadcast are below. Learn more about the speaker and his topic here.
The Electrochemical Society hosted Kyle C. Smith’s live webinar, “Intercalation-Based Desalination and Carbon Capture for Water and Climate Sustainability” on May 7, 2025. A live Question and Answer (Q&A) session followed. Dr. Smith’s written answers to questions not addressed during the broadcast are below. Learn more about the speaker and his topic here.
Note: Registration is required to view the webinar replay.
Q&A
How far is this method successful in removing other ions such as Li, K, etc.,?
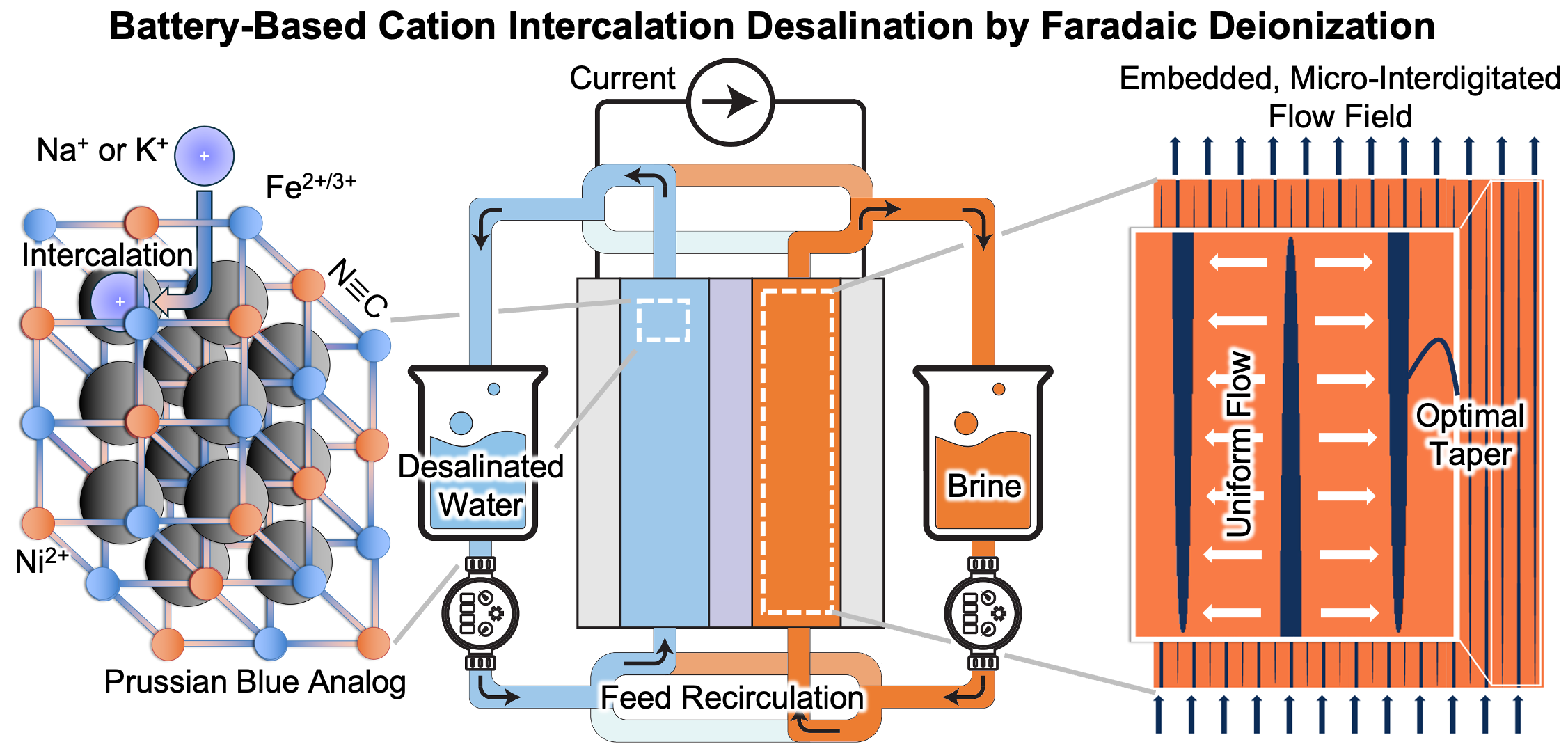
In our recent yet-to-be-published work [Do and Smith, in review (2025)], we have successfully tested this process using feeds comprised of 3 g/L Instant Ocean® synthetic salt that includes Na+, K+, Mg2+, and Ca2+ cations and that is representative of brackish water resulting from seawater intrusion into coastal groundwater. Using symmetric nickel hexacyanoferrate electrodes, all monovalent cations were shown to be readily removed, whereas divalent cations were rejected altogether. Generally, Prussian blue analogues exhibit selectivity bias toward monovalent cations with small hydration shells. In other work of ours using NASICON electrodes with such an architecture [Shrivastava, Do, and Smith, ACS Appl. Mater. Inter., 14, 30672 (2022)], the process was shown to selectively remove Na+ and Li+ cations, while rejecting K+, NH4+, Mg2+, and Ca2+.
Can flow-electrode capacitive deionization (FCDI) and salinity gradient energy (SGE) systems be integrated with redox-active (intercalation) materials to simultaneously achieve water desalination, energy storage, harvesting?
There isn’t a fundamental barrier that prevents intercalation materials from being used in FCDI or in SGE systems. However, FCDI may present unique challenges in using it with specific intercalation materials. Previous work incorporating LiFePO4 and LiTi2(PO4)3 into aqueous semi-solid flow batteries [Li, Smith, Dong, Baram, Fan, Xie, Limthongkul, Carter, and Chiang, Phys. Chem. Chem. Phys., 15, 15833 (2013)] suggests that this is possible, however. It is also possible to operate the present architecture that we have used for desalination to generate energy via a salinity gradient. Past SGE systems have used symmetric copper hexacyanoferrate electrodes [Kim, Rahimi, Logan, and Gorski, Environ. Sci. & Tech., 50, 9791 (2016)].
NASICON materials have much higher structural and chemical stability than Prussian blue analogs. Can you comment on which class of compounds will be more effective for intercalation-based desalination?
We demonstrated recently that the NASICON material Na2TiV(PO4)3 functions well in desalination experiments [Shrivastava, Do, and Smith, ACS Appl. Mater. Inter., 14, 30672 (2022)]. This ability of Na2TiV(PO4)3 is owing to its moderate potential for Na+ intercalation. Despite its modest specific capacity as compared with Na3V2(PO4)3 and NaTi2(PO4)3, Na2TiV(PO4)3 is less prone to O2 evolution and V4+ dissolution than is Na3V2(PO4)3 , and it is less prone to self-discharge via dissolved O2 than is NaTi2(PO4)3. In the same work [Shrivastava, Do, and Smith, ACS Appl. Mater. Inter., 14, 30672 (2022)], we showed that streamwise state-of-charge gradients are minimized for a given degree of desalination when the active material intercalates cations without a miscibility gap. Among NayTixV2-x(PO4)3-type NASICON materials, Na2TiV(PO4)3 uniquely intercalates cations via a solid-solution mechanism, thus minimizing the extent of feed recirculation that is needed to overcome streamwise state-of-charge gradients. These features of Na2TiV(PO4)3 are thus desirable for any NASICON material, in addition to maximizing specific capacity and rate capability.
How many cycles can your system be effective with, while doing desalination?
We have used our nickel hexacyanoferrate electrodes that contain embedded flow fields [Do, Reale, Loud, Rozzi, Tan, Willis, and Smith, Energy Environ. Sci., 16, 3025 (2023)] for more than 200 cycles with no apparent drop in specific capacity. We note that in certain experiments, we have observed a reduction in the full-cell capacity obtained with flow cells over a sufficient number of cycles. After cycling the associated electrodes in a three-electrode flooded cell, capacity is shown to be regenerated, thus implicating mechanisms other than active-material degradation with the associated reduction capacity.
Learn more about upcoming ECS Webinars and review our previous webinar recordings.
Interested in presenting in the ECS Webinar Series?
Email your presentation title and abstract to education@electrochem.org for consideration.