The Electrochemical Society hosted Thomas S. Miller’s live webinar, “Electrochemical Atomic Force Microscopy of Battery Interfaces,” on March 27, 2024. Dr. Miller took audience questions during a live Question and Answer following the presentation, then kindly answered in writing, questions not answered during the broadcast. See his responses below.
The Electrochemical Society hosted Thomas S. Miller’s live webinar, “Electrochemical Atomic Force Microscopy of Battery Interfaces,” on March 27, 2024. Dr. Miller took audience questions during a live Question and Answer following the presentation, then kindly answered in writing, questions not answered during the broadcast. See his responses below.
NOTE: Registration is required to view the webinar replay.
Q&A
How could you probe the Zn flow battery electrode if it is a closed system and to probe the electrode with the AFM you need an open cell to scan the electrode with the tip?
In this work, we designed an EC-AFM flow cell that allowed electrolyte to flow across and through the electrode. The cell was open at the top to allow cantilever access, so electrolyte was pumped from the cell at the same rate that it was pumped in, hence the electrolyte level remained constant. Find more details at https://doi.org/10.1021/acsami.3c03785.
Is it possible to study real battery electrodes by AFM? Or only model system electrodes?
It is certainly possible, but the balance between roughness and resolution will define the results obtained; rougher samples (as usually found with commercial anodes/cathodes) will limit the nanoscale details it is possible to resolve.
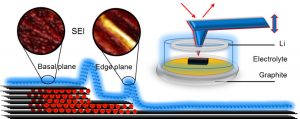
What is the mechanism that causes thicker SEI growth at step edges?
Thicker SEI grows at step edges as these are the sites towards which there is a higher Li-ion flux, as ions must enter between the layers via these steps. This increased flux, along with higher step reactivity, leads to additional SEI accumulation.
Battery technology has been around for centuries. Why are these sophisticated battery studies and investigations only starting now?
The increased interest in advanced batteries is largely driven by the increased interest in sustainable technologies and the increasing desire for portable personal electronics.
Is the liquid electrolyte in the operando cell a problem for AFM measurements since the cantilever will be physically contacting the samples? Especially for the young’s modulus measurement?
The liquid electrolyte poses some problems, but AFM imaging in liquids is a common and hence controlling these issues is increasingly uncomplicated with system/software improvements. Force measurements must be carefully calibrated when performed in liquids, but this is also quite common outside of electrochemical AFM.
Is there a way to use AFM to define the chemical composition of SEI? Or must it be done with other techniques such as XPS?
It isn’t currently easily possible to directly determine the chemical composition (although there is work being done to integrate techniques such as Raman spectroscopy with AFM). However, mechanical properties measurements can allow some degree of differentiation between organic (polymeric) and inorganic SEI components as there is a big difference in Young’s modulus. We explore this in our recent publication: https://doi.org/10.1021/acsnano.3c10208.
The SEI ideally will grow on the whole surface of anode, however, the AFM can only measure a small area on it, how could you justify this selection of area is representative enough for the entire anode since the current density on anode surface will surely be non-uniform during a operando experiment?
By using model anodes, such as HOPG, we can be confident of a consistent electrode structure. Hence we can be sure that a small area is representative. Scanning a number of areas of the electrode post cycling helps confirm consistency. We have then worked to compare the performance of these model electrodes and more representative battery materials: https://doi.org/10.1021/acsami.0c11190.
If operando AFM is difficult for our setup and we have to do it in ex situ mode, what would you recommend to pay extra attention to ensure the data quality without too much damage during harvest/setup?
Ex situ experiments are difficult as the SEI is very delicate and prone to damage during cell deconstruction and rinsing (to remove excess salt). However, these studies are likely to be less problematic for more stable surface structures, e.g., metal deposits.
Is it possible to conduct in situ/operando AFM for all solid state batteries to probe the SEI/CEI morphology?
While studies such as these have been performed, they are very complicated as it isn’t possible to access the electrode/electrolyte interface directly using the scanning probe. We discussed some of these studies in a recent review: https://doi.org/10.1002/aenm.202101518.
How does the HOPG’s particle size and SEI formation change when combined with a full Li-ion cell? Could you comment on the examples of operando AFM where the electrode surface morphology is studied in a full cell that is close to the real LiBs technology?
To date, I can’t think of any studies that use cells that are close to real LIB batteries, as the requirements of the AFM system are not yet well aligned. This is a priority area of study. We compared the progress of EC-AFM and other in situ/operando techniques in a recent review: https://doi.org/10.1002/aenm.202101518.
What is the difference of NMC622 vs. NMC811 degradation using AFM observation?
We haven’t studied this difference using AFM, but it is commonly acknowledged that NMC811 suffers from more severe degradation than 622. This would be an interesting AFM comparison for the future.
Why not work in tapping mode, as contact mode is a bit harsh on soft materials?
In our work we use peak-force tapping mode, in which force curves are performed at each point of contact. Using this mode means tip-surface force is carefully controlled.
Is it possible to study the properties of SEI in air?
The SEI is known to be air sensitive, so this would be difficult.
Is it possible to study the properties of SEI in air after operation?
This may be possible, but results would be complicated to interpret as the SEI would suffer from both damage during processing (rinsing off electrolyte salt) and reaction with air. This is why we believe operando measurements are important for SEI studies.
Do you have a video showing how EC-AFM works?
I don’t have a video, but we discussed how EC-AFM works in a recent review https://doi.org/10.1002/aenm.202101518.
What is the difference between AFM and EC-AFM? Which one is most cost efficient?
AFM is simply atomic force microscopy, a widely used and relatively cost-effective characterization method. As electrochemical AFM is more specialized it will be more expensive (as AFM + electrochemical control is needed, plus a glovebox for air free work).
How could we distinguish the degradation of HOPG or solid electrolyte at higher voltages to the change in surface morphology due to Li insertion?
Li inserts into HOPG at low voltages (vs Li/Li+), so will occur after SEI has formed. De-insertion will cause a morphology change of the HOPG, but graphite is known to only suffer from ~10 percent volume change during lithiation so this change is very subtle.
Images from Li battery work show artifacts coming from the AFM probe may not be representative of the true surface; any comment on that?
Operando scanning will make scanning artifacts more likely, as the surface being studied along with the electrolyte structure, will be changing dynamically. Nonetheless, the artifacts most commonly observed are skipped lines, which appear clearly as horizontal lines on the images and can hence be easily distinguished from real surface change. Repeat measurements are always vital to ensure consistency.
Could you tell changes of Li stripping/plating using electrochemical AFM while the SEI is existing?
This would depend on the roughness of the Li deposits and thickness of the SEI. But researchers have studied Li using EC-AFM and the Li structure is usually resolvable.
How do you control the electrolyte’s solvent evaporation in the open cell batteries? How does it impact the measurement?
Evaporation is certainly a challenge we face, but as there is a significant excess of electrolyte in the cells used, this problem is minimized. We also use flexible cell caps to minimize evaporation. Nonetheless, as we hope to move to leaner electrolyte conditions, this will become an increasing challenge in the future.
In your Li-ion batteries part, do you have any idea how much adding transition metal ions changes the chemical composition of the SEI layer?
Further details on the chemical composition can be found in the full paper; we study this using electrochemical quartz crystal microbalance measurements and XPS: https://doi.org/10.1021/acsnano.3c10208.
How stable are the cantilevers in the electrolyte? Any corrosion of the tips?
We have found no negative influence of the electrolyte on the cantilevers used.
The images are collected during potential sweeping. Are there technical reasons why you do not use constant-current/potential sequence for testing?
No technical reason here; we chose to use CV /LSV as it means we can more easily link cell voltage (and hence the processes occurring) to the dynamic (line-by-line) morphology/mechanical properties observed. With zinc batteries we have more commonly used constant-current measurements.