The Electrochemical Society hosted Dr. Wesley Dose’s live webinar, “Challenges Facing Li-ion Battery Electrolytes and High-energy Cathodes,” on September 21, 2022. Dr. Dose took audience questions during a live Question and Answer session at the end of the presentation. He kindly answered, in writing, questions not answered during the broadcast. Find these responses below.
The Electrochemical Society hosted Dr. Wesley Dose’s live webinar, “Challenges Facing Li-ion Battery Electrolytes and High-energy Cathodes,” on September 21, 2022. Dr. Dose took audience questions during a live Question and Answer session at the end of the presentation. He kindly answered, in writing, questions not answered during the broadcast. Find these responses below.
NOTE: Registration is required to view the webinar.
Q&A
1. How would changing ethylene carbonate (EC) to fluoroethylene carbonate (FEC) affect the reactivity with high-Ni LiNixMnyOzO2 (NMC) cathodes?
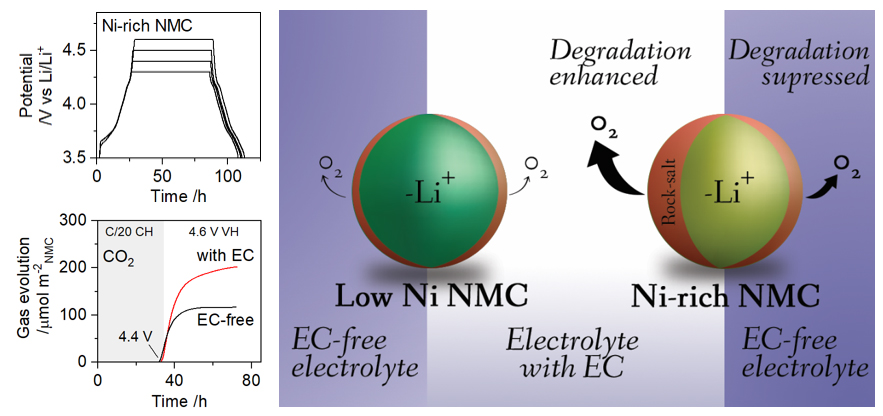
One important implication of our work (W.M. Dose et al., ACS Appl. Mater. Interfaces, 14, 13206, 2022, https://doi.org/10.1021/acsami.1c22812; W.M. Dose et al., ACS Energy Lett., 7, 3524, 2022, https://doi.org/10.1021/acsenergylett.2c01722) is that tuning the electrolyte composition has the potential to significantly alter the extent of lattice oxygen release from charged NMC cathodes, and hence the degree of cathode and electrolyte degradation. The relative reactivity of many of the electrolyte solvents/additives commonly used in Li-ion battery (LIB) electrolytes still needs to be determined—like FEC, but also others such as linear carbonates. It will be interesting to see future investigations, both experimental and modelling, that explore this avenue in depth.
2. What is the role of Ni content in the decomposition of EC?
Our findings highlight the detrimental effect of EC, a core component in conventional LIB electrolytes, when the NMC cathode is charged above the onset potential of lattice oxygen release. This onset potential is a function of the cathode Ni-content, with higher Ni giving rise to a lower onset potential. We believe that the main effect from Ni-content is likely related to this lower onset potential for lattice oxygen release, which subsequently initiates other reactions including electrolyte degradation.
3. How detrimental is EC for cells kept below 4.2 V, the traditional upper limit?
This depends on the Ni-content of the cathode used in the cell, and hence the onset potential for lattice oxygen release. If the cell is cycled to an upper cutoff voltage (UCV) that maintains the cathode potential below that for oxygen release, then one could reasonably expect little (or no) detrimental effect from EC in the electrolyte. The caveat being that the reported onset potential will likely depend on the sensitivity of the detection method—small amounts of oxygen loss/electrolyte breakdown may go unnoticed in some experimental setups, but could nonetheless adversely affect the cell performance.
4. Do we see similar degradation mechanisms with other cathode chemistries?
Many of the degradation processes that we investigate, such as impedance rise and transition metal dissolution/deposition, are common to other cathode chemistries—even those that do not release lattice oxygen. For example, LIBs with a lithium iron phosphate (LiFePO4, LFP) cathode also experience performance fading related to these processes. Our work highlights the incompatibility between conventional EC-containing electrolytes and LIB cathodes charged above the onset potential for lattice oxygen release, with a focus on the degradation processes initiated and/or accelerated by lattice oxygen release. In our investigations to date, we have focused on a Ni-rich NMC cathode (LiNi0.8Mn0.1Co0.1O2, NMC811); however, our findings have important implications for other cathode materials that release lattice oxygen in the course of cycling. This includes other NMC compositions (particularly those with high Ni content), as well as several next-generation cathode materials, such as Li-/Mn-rich and disordered rock-salt cathodes.
5. You mentioned that some degradation processes are more related to the extent of cathode delithiation rather than the cycling upper cutoff voltage. Can you define delithiation?
The extent of delithiation is the fraction of lithium that has been extracted from the cathode material—i.e., x in Li1-xMO2, where M is one or more transition metals. From the electrochemistry data, the extent of delithiation in the first charge can be calculated by dividing the charge capacity by the theoretical capacity of the cathode material. (The theoretical capacity can be calculated based on the theoretical amount of lithium available.) Note that this calculation assumes that all of the charge capacity arises from lithium extraction from the cathode—the validity of this assumption should be assessed on a case-by-case basis.
6. Could you comment on the Ni-rich cathode interface with dimethyl carbonate (DMC)? How does it compare to the ethyl methyl carbonate (EMC) interface?
Refer to the answer to question 1.
7. Which analytical technique/method is used to measure the gas release quantitatively and qualitatively?
In our work, we used online electrochemical mass spectrometry (OEMS). Refer to refs. (W.M. Dose et al., ACS Appl. Mater. Interfaces, 14, 13206, 2022, https://doi.org/10.1021/acsami.1c22812; W.M. Dose et al., ACS Energy Lett., 7, 3524, 2022, https://doi.org/10.1021/acsenergylett.2c01722) for additional details of this technique.
8. Is lattice oxygen release necessary for solvent oxidation? I assume that the solvent may be electrochemically oxidized even on inert metal surfaces such as Pt at those high potentials.
The electrolyte components may by oxidized electrochemically above the onset potential for this process. A study by Solchenbach et al. (S. Solchenbach et al., J. Electrochem. Soc., 165, A3022, 2018, https://doi.org/10.1149/2.0481813jes) shows that the onset potential for electrochemical oxidation of 1.5 M LiPF6 in EC is 4.95 V vs. Li/Li+. This is well above the 4.6 V cutoff used in our investigations. While we do not rule out a small contribution from electrochemical oxidation (e.g., an upper limit for the contribution is calculated in ref. (W.M. Dose et al., ACS Appl. Mater. Interfaces, 14, 13206, 2022, https://doi.org/10.1021/acsami.1c22812)), we do not believe that it is the main cause for the electrolyte degradation that we observe.
9. Can you explain how to recognize rock-salt versus spinel structure in the transmission electron microscope (TEM) images?Crystal structures can be identified directly from high-resolution transmission electron microscope (HRTEM) images using crystallographic image processing. We applied a fast Fourier transformation (FFT) to specific regions of the HRTEM images, from which the crystal structure (i.e., layered vs. spinel-like vs. rock-salt-like) can be easily identified.
10. Do you think we should replace EC with something else or just eliminate EC from the system?
Our work has highlighted the challenges facing EC-containing electrolytes due to the degradation of the cathode and electrolyte that ensues when the cathode is taken above the onset potential for lattice oxygen release. Based only on this, removing EC may seem like a logical approach. However, EC currently plays several important roles in conventional LIB electrolytes. These include (but are not limited to) formation and repair of the anode solid electrolyte interphase (SEI), inhibiting severe gassing in the case of lithium plating, and providing high ionic conductivity. This highlights the conflicting needs of the anode and the cathode. Any low-EC or EC-free electrolytes that are developed will have to be rigorously tested in order to ensure they provide all-round good performance. This includes not only capacity retention, but also rate performance, wide operating temperature, and excellent safety characteristics.
11. Is it solely EC that creates these problems at high-nickel cathodes, or also FEC, propylene carbonate (PC), cyclic carbonates in general?
Refer to the answer to question 1.
12. What kind of properties will be lost by removing EC?
Refer to the answer to question 10.
13. What practical tips can you give to avoid electrolyte decomposition? Change electrolytes?
It is clear from our work that electrolyte development has the potential to enable a step change in the battery performance. Further, the need to suppress lattice oxygen release from the cathode, which initiates many other degradation processes, also suggests that materials-based solutions may be important. It is hoped that our work encourages researchers in the field to explore such solutions (e.g., novel electrolytes, material coatings/dopants, particle morphologies, etc.) for their ability to suppress, or even inhibit, oxygen release. Our hypothesis is that such functionality will lead to improved cell life times for LIBs with high-capacity Ni-rich cathodes, as well as for LIBs with other next-generation cathode materials.
14. Are the two degradation mechanisms of EC only for NMC cathodes, or could it happen even for lithium iron phosphate (LFP) cathodes?
The two degradation mechanisms for EC that were discussed in the webinar were (i) that proposed by Shao-Horn and co-workers (L. Giordano et al., J. Phys. Chem. Lett., 8, 3881, 2017 https://doi.org/10.1021/acs.jpclett.7b01655; Y. Zhang et al., Energy Environ. Sci., 13, 183, 2020, https://doi.org/10.1039/C9EE02543J), and (ii) that proposed by Gasteiger and co-workers (J. Wandt et al., Mater. Today, 21, 825, 2018, https://doi.org/10.1016/j.mattod.2018.03.037; A.T.S. Freiberg et al., J. Phys. Chem. A., 122, 8828, 2018, https://doi.org/10.1021/acs.jpca.8b08079). The degradation of EC on NMC surfaces has been observed by Shao-Horn and co-workers for Ni-rich NMC (i.e., for NMC811) above ~3.9 V vs. Li/Li+, but was not observed for LiNi0.33Mn0.33Co0.33O2 (NMC111) even up to 4.8 V vs. Li/Li+. Based on the onset potential alone, we believe that it would be unlikely for this degradation process to be active in LIBs with a LFP cathode, given these cells typically have an UCV of around 3.7 V. The mechanism for EC degradation proposed by Gasteiger and co-workers is initiated by the release of lattice oxygen, in the form of singlet oxygen, which reacts with EC. As the LFP cathode does not release oxygen under normal cycling conditions, we would not expect this degradation process to be active in LIBs with a LFP cathode.