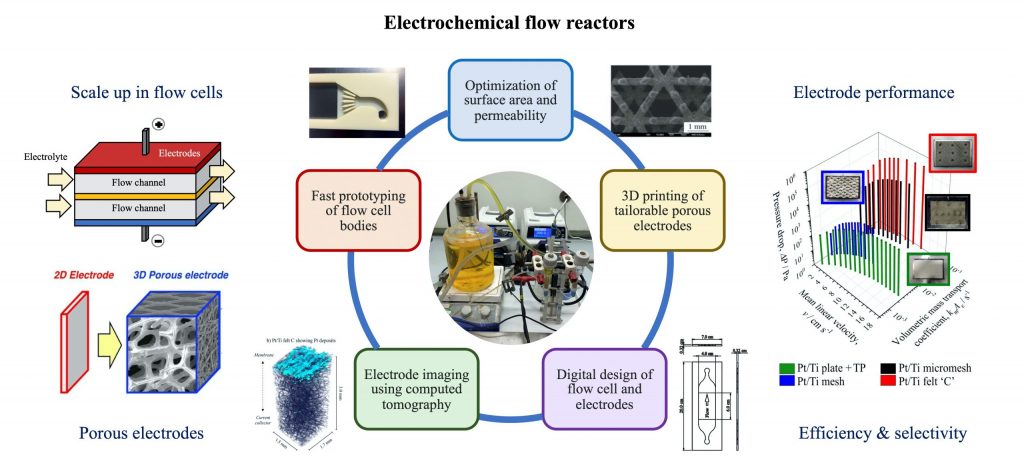
The Electrochemical Society hosted Dr. Luis Fernando Arenas’ live webinar, “Flow Cells: Advanced Electrodes via 3D Printing and Tomography,” on July 20, 2022. Dr. Arenas took audience questions during a live Question and Answer session at the end of the presentation, and was kind enough to answer, in writing, questions he was not able to get to during the broadcast. Read his responses to these questions below.
View Dr. Luis Fernando Arenas’ WebinarNOTE: Registration is required for viewing
Q&A
This question relates to the electrodeposition of the porous electrode. Is the Watt’s bath stirred during deposition? How homogeneous is the coating?
Our first 3D printed stainless steel electrodes were coated with nickel in a flow cell. We used this unorthodox approach for convenience and to learn how feasible this method was, which is not new. The Watts bath was continuously flowing through the porous electrode at an average velocity of 8 cm per second. SEM showed that the coating was uniform at the external face of the electrodes, but we couldn’t analyze the interior of the porous material without destroying it. The electrodes were 4 mm thick, and from experience we can assume that the whole material was coated, though surely with smaller thickness closer to the current collector and in surfaces not facing the counter electrode.
You mentioned “pressure drop” several times. Does it mean the pressure drop in the electrolyte or the electrode?
We are talking about the head loss that the flowing electrolyte experiences as it finds the porous material on its way. To put it in another way, if we locate a pressure sensor in the electrolyte path before it reaches the electrode and another after it, the first pressure will be larger than the second one. This is called pressure drop and can be related to a friction factor or to a permeability.
What are your thoughts on SLA 3D printing for specialized resins with tuned-up electrical properties?
This would be a creative way to produce polymer-based 3D printed electrodes. A filler would provide the conductivity in the bulk of the material and supply a surface for electron transfer at the interphase with the electrolyte. Chemical stability would be key to performance.
Have you ever compared your 3D-printed electrodes with 3D fix/fluidized bed electrodes? Made from active carbon?
No, we haven’t. In our case, we need self-standing electrode materials. But I would like to try ways in which we could combine, for example, the impressive surface area and performance of a fluidized bed of small carbon particles with porous electrodes.
Do you know how economically viable 3D printed electrodes are for mass production?
We would like to study this question in order to provide figures. But for now, it is easy to see that the energy consumption and slow throughput of 3D printing is not really compatible with mass production, except maybe for small sensors. Our objective is to exploit the advantages of 3D printing in fast prototyping of better mass-produced materials or in developing applications where the benefits of a tailored electrode surpass the cost of making a small number of pieces.
Was there an “important” difference in the printing times for the different geometries?
The geometry that took longer to print was one with “circular” open-cell structure. It needed around twice the time than one with “triangular” shapes.
How long does it take to print one electrode?
If I remember correctly, in the case of the stainless steel electrodes, each took about 4 hours. The titanium electrodes were smaller, so less than that.
What is the potential reason that a rectangular shaped pore is more efficient (permeability and surface area) than the others?
I think that you are talking about the rectangular open-cell structure grade 30 ppi. Its surface area was larger than the other materials because its pores were the smallest. All the other structures were 20 ppi, as limited by our resources. But the rectangular 30 ppi structure was not more “efficient” in terms of permeability, its value being in fact quite low. This is why we say that the triangular 20 ppi was “efficient” since it combined large surface area with a reasonably high permeability.
What are the main limitations regarding 3D printing technologies? Is resolution essential? What about mechanical stability and cost?
The main limitation that I see is that, for most applications, off-the-shelf electrode materials have lower costs. Another is the challenge of uniform catalytic coatings. The resolution is not extremely important, since we are interested in slightly rough surfaces, similar to the sandblasted titanium used in the production of industrial Pt/Ti and DSA electrodes. Mechanical stability of our 3D printed materials is excellent; you can stand on them without problem.
How was the behavior of pressure drop on the zinc side due to plating during battery charging?
This question is related to the operation of the zinc-cerium flow battery. (During my talk, I used it as a case study on the development of porous electrodes for the cerium half-cell.) Actually, we didn’t measure the pressure drop over the half cells in that device. However, the electrodeposition of zinc took place on a planar electrode at a thickness of fractions of millimeters. Hence, I would expect that the pressure drop didn’t change much during charge. However, if the electrodeposition was unusually thick or if it took place on a flow-through porous electrode, then you would see a higher pressure drop. Some of my colleagues recently published a model of how the coating thickness on a carbon foam would affect the operation of a lead flow battery.
Printing metal requires high temperature; how can you print SS?
Correct. Basically, in order to “print” a metal, a laser beam is directed to a bed of fine metal powder, melting it locally in the points that follow a digital design. This process repeats many times and creates an object layer by layer, until it is finished. If the precursor powder is, for example, stainless steel, then the resulting piece will be a “3D printed stainless steel.”
What are the electrolytes on either side of the membrane in Ce-Zn cells? Are Ce(III) and Ce(IV) coordinated with other molecules in the solution?
This is another question about the zinc-cerium flow battery. The cerium ions are coordinated with methanesulfonate ions. Both electrolytes are methanesulfonic acid solutions.
Is there any study on the role of roughness? Other printing techniques such as SLS are expected to produce more rough 3D printed objects.
We haven’t been able to measure the roughness of the 3D printed electrodes, but our imaging techniques clearly show it. Roughness for an electrode means extended surface area and some degree of mass transport enhancement. It would indeed be interesting to see how SLM and SLS differ on producing such surfaces. I am sure that a virtual profilometry can be performed digitally on the computed tomography renderings, even in the interior of the porous structure.
If you compare compressed graphene plaques and your electrodes, which will be more efficient?
This means what do you mean by “efficiency.” But a plaque will have a smaller geometrical surface area than the one we would typically find in a three-dimensional porous electrode of the same projected surface area. Plus, the surface microporosity of a compressed graphene plaque, which might be larger in total, might not make so much difference in a concentrated, flowing electrolyte when compared to a smooth carbon-composite plaque in terms of limiting current. On the other hand, electrode materials have different activation properties and selectivity for a given reaction, and in this case the right catalytic surface can make an important difference in an actual current efficiency regardless of the shape, if that was the question.
Can you comment on other 3D printing techniques such as robocasting and the potential for printing multi-material devices and interfaces?
Robocasting is a type of additive manufacturing that produces an object from filaments that solidify immediately. The difference is that it is used typically for ceramics and might have a limited use in creating electrodes. But this is useful in heterogeneous catalysis. On the other hand, we have talked about printing whole electrochemical flow cells in our publications. Flow frames, turbulence promoters and separators are printed in impermeable polymer, gaskets in flexible polymers or resins and electrodes in metal or conductive polymer. The concept is possible.
What is the capacity of a 3D printed electrode? Are there any tradeoffs?
I will take capacity as a synonym of benefits. 3D printed electrodes might be useful in fast prototypes of electrochemical devices. Or they could be useful in providing interesting problems for mathematical modeling which could help us find the limits of these materials and design better mass-produced electrodes—or perhaps in situations where a device needs to be produced unexpectedly in remote sites, like in space. The tradeoff might be the cost and time of production, against the advantage of having a tailored material at will.
What are the emerging and promising flow cell batteries currently? Also, which is promising to be commercialized in the future?
In regard to flow batteries, vanadium systems work very well but the price must continue to decrease in order to become widespread. Zinc-bromine is available right away and is very competitive for some applications. Much work is being done at the moment on flow batteries based on organic molecules. Some anthraquinones might have a good chance in couple to hexacyanoferrate if they can remain at the same concentration for years. But presently most organic redox molecules remain too unstable or expensive for commercial use. Some companies are trying to change this.
You mentioned using the active surface area to calculate the electrode performance factor. When calculating the active surface area, did you consider how two electrodes of the same overall surface area will have different active areas if they are structured differently, as flow will distribute differently through it?
Maybe it is better to give more details about the performance factor. It actually contains the product of active surface area times mass transport coefficient. They are kept together because it is difficult to separate them experimentally. And the performance factor is calculated for the volume in space that contains the porous electrode. So, for the same projected surface area, the factor considers that the electrode differs in their total active surface area. Since the mass transport coefficient is also there, changes due to different flow are also taken on account. This factor is very useful, but, of course, a complete description of the reaction environment over the whole range of current density and reaction rate regimes requires modeling tools.
With the open cell architectures, did you try creating them with all the same overall surface areas, and seeing how performance compares?
No, presently it would be difficult for us to create all the structures with the same overall surface area. But I see the point, it would be very interesting to know the effect of fluid flow and mass transport if the surface area is fixed. We had to start with the geometry and open-cell size and measure the resulting characteristics later. The next steps could involve more complex questions.
Is there a way to 3D print the electrodes using carbon fiber?
Yes, there are some papers on that already. As in the other questions, thick might involve printing thin, conductive fibers. There are different approaches and we are also trying to move in that direction. But don’t think printing fibers is remotely close to compete with the continuous mass production of hundreds of meters of carbon felt in a factory.
In our experiment, we did appreciate the benefit of catalyst coated carbon cloth porous electrode due to the increased surface area for water contaminant removal. However, we also observed those electrodes would have large background current (both capacitive and faradaic) and it caused trouble for overall current efficiency, and difficulty in performing CV on those electrodes. We also saw it hard to determine the background reactions happening on the carbon cloth electrodes. Do you have any suggestions on how to address these difficulties either particular to carbon cloth or in general for porous electrodes?
Indeed, carbon cloth is another common porous electrode material. The very large area of a porous electrode is not beneficial to get textbook cyclic voltammograms. Even though cyclic voltammetry can give you some valuable information about the material, and it is certainly possible to perform on these electrodes, this technique is not adequate to characterize the performance of an electrochemical flow reactor. Fractional conversion over time, single pass conversion along electrode potentials, and cell voltage monitoring might be more useful in practice. About the faradaic background current, assuming potentiostatic control, you might be observing the reduction of the oxidized functional groups on an activated carbon felt, which might decrease over time. But it is difficult to tell without knowing more about the composition of the electrolyte, type of cloth, catalysts, and experimental conditions. In general, I would suggest to make sure that the cell components have low resistance, good contacts. and minimum inter-electrode and membrane-electrode gaps.
Those are metal-based electrodes. Do you think polymer electrodes will work as well as metals?
In terms of materials, I think it is possible to 3D print polymers in an effective way, at least as much as our examples. One would have to consider the conductivity and chemical stability of the resulting electrodes, but compositions and processes could be worked out. The reason we used metal electrodes is because they are often found in industrial operations, where they have to be robust and durable. But there are opportunities for the many cases where carbon-based electrodes are preferred.
What technique is used for imaging?
We used optical microscopy, SEM, EDS, and X-ray computed tomography. The raw images from tomography can be used as 2D radiographies, but, of course, it is more convenient to create 3D digital renderings from the objects, which the software can then analyze in various ways.
How will the pore size distribution effect the performance? Will the porous electrode be better with mesopores?
In theory, the pore sizes are very uniform in our structures, since the open-cells are intended to be identical to each other. This is in contrast to foam structures or packed beds, which might have an ample pore size distribution. But yes, different pore size distributions would change the performance to a certain extent. I have to clarify that for our purposes, “pores” refer to fluid flow-through porous media. In materials science, we use the terms meso- and nanopores to describe porosity at a much smaller scale. However, to answer the question, mesopores at the surface of these electrodes can have a minor, but significant, contribution to the electrochemical active surface area in a flow cell. This was shown by a paper on the mass transport to a nanostructured nickel electrodeposit in a flow channel.
Will the catalyst loading be limited when using printing technology to produce an electrode? How can the heterogeneous catalyst be loaded when used for other applications such as metal-air battery (Li-O2, Li-CO2 battery)?
As with any porous electrode material, its ability to support a surface catalyst depends on chemical affinity, pre- and post-treatment, thickness of the catalyst, and application method. Thick porous materials are often problematic. In addition to the standard methods, a heterogeneous catalyst can be loaded using 3D printing by embedding the precursor material with it, for example in a powder or resin.
Dr. Luis Fernando Arenas
Dr. Luis Fernando Arenas is a Humboldt Research Fellow at Technische Universität Clausthal where he collaborates with Prof. Thomas Turek on the development of organic flow batteries. He is also a Visiting Academic at the University of Southampton (UOS) and Early Career Representative at the Electrochemical Technology Group of the Society of Chemical Industry. After completing a BSc in Chemistry and MSc degree in Chemical Technology at the Universidad Autónoma de Coahuila, he pursued doctoral studies in Electrochemical Engineering at UOS. There he focused on zinc-cerium flow batteries under the supervision of Profs. Carlos Ponce de León and Frank C. Walsh, graduating in 2017. He was a Research Fellow at UOS, developing PEM fuel cells and electrochemical hydrogen pumps (2017-2019). Dr. Arenas has also been a private contractor for the development of new organic- and electrodialysis-based flow batteries for INEEL (Instituto Nacional de Electricidad y Energías Limpias) and consultant for the chlor-alkali industry (2019-2021). Additional interests include 3D printed electrodes, computed tomography, electrodeposition of noble metals, porous electrodes, and sustainable electrochemical engineering. He became an ECS member in 2014. His research has resulted in 31 peer-reviewed papers, four book chapters, six conference papers, and 17 posters, as well as 40 talks, seven of them by invitation (Google Scholar: 1018 citations, h-index 17). Dr. Arenas received the 2019 Schwäbisch Gmünd Prize for Young Scientists for excellence in electrochemical surface technology.