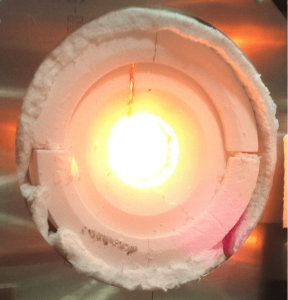
By concentrating sunlight into reactors, H2O and CO2 can be split to form liquid fuels.
Image: The Conversation/David Hahn
The sun produces an astronomical amount of energy each day, but scientists and engineers are still trying to better understand how to convert that energy into an efficient, usable form. Recently, work in photovoltaics deals with utilizing different materials, new arrangements of cell components, and interdisciplinary work to improve efficiently levels. However, a new and exciting area of photovoltaics is now rising in the ranks: turning sunlight into liquid fuels.
With this new development on the rise, the possibility of one day filling our cars with solar-generated fuel is on the horizon.
Researchers are giving more attention to the production of solar fuels because energy conversion and storage and simultaneously covered under one technique. It will give solar energy a wider scope due to more utilization opportunities, whereas conventional photovoltaic energy is only being used for one-third of the day when sunlight is at its peak.
Currently, the greatest roadblock lies in commercialization of the man-made solar fuels due to the substantial amount of energy it takes to break down stable CO2 and H2O molecules.
However, researchers are also exploring aspects of artificial photosynthesis through electrochemistry to help produce efficient, affordable man-made solar fuels.
Further material from the ECS Digital Library:
Read more about processes and current projects on The Conversation.
PS: Watch Ralph Brodd, a pillar of electrochemical science and technology with over 40 years in the electrochemical energy conversion business, talk about the future of the energy infrastructure and how it has transformed over the years.