Presenters: Lennart Reuter and Leonhard J. Reinschlüssel
Technische Universität München (TUM)
Date: August 6, 2025
Time: 1000-1100h ET
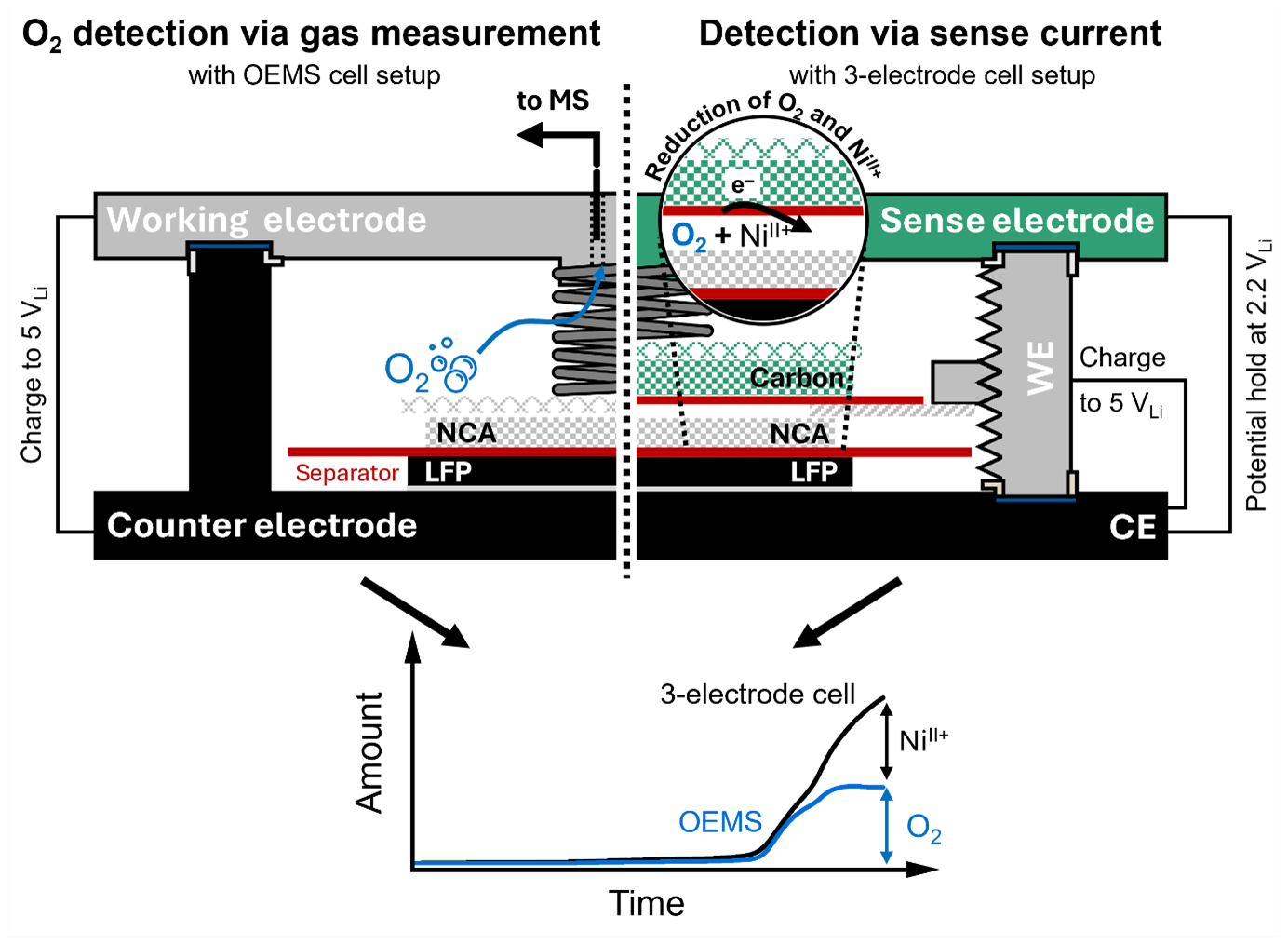
Detecting parasitic side reactions originating both from the cathode active materials (CAMs) and the electrolyte is paramount for developing more stable cell chemistries for Li-ion batteries. This talk presents a method for the qualitative analysis of oxidative electrolyte oxidation, as well as the quantification of released lattice oxygen and transition metal ions (TM ions) from the CAM. It is based on a three-electrode cell design employing a Vulcan carbon-based sense electrode (SE) that is held at a controlled voltage against a partially delithiated lithium iron phosphate (LFP) counter electrode (CE). At this SE, reductive currents can be measured while polarizing a CAM or carbon working electrode (WE) against the same LFP CE. In voltammetric scans, we show how the SE potential can be selected to specifically detect a given side reaction during CAM charge/discharge, allowing, for example, to discriminate between lattice oxygen, protons, and dissolved TMs. Furthermore, it is shown via on-line electrochemical mass spectrometry (OEMS) that O2 reduction in the here-used LP47 electrolyte consumes ~2.3 electrons/O2. Using this value, the lattice oxygen release deduced from the three-electrode setup upon charging of the NCA WE is in good agreement with OEMS measurements up to NCA potentials >4.65 VLi. At higher potentials, the contributions from the reduction of TM ions can be quantified by comparing the integrated SE current with the O2 evolution from OEMS.
Benefits of attending the webinar
Learn about:
- Required considerations;
- How to use reductive currents for qualitative and quantitative species analysis.
Presenters
Lennart Reuter is a PhD student in the Chair of Technical Electrochemistry Gasteiger research group at TUM. Lennart’s research focuses on the interfacial processes in lithium ion batteries that govern calendar life, cycle stability, and rate capability. He advanced online electrochemical mass spectrometry (OEMS) technique to investigate gas evolution mechanisms from interfacial side reactions at the cathode and anode. His work also explored how SEI formation and graphite structural changes affect Li⁺ transport, using impedance spectroscopy and complementary analysis techniques.
Leonhard J. Reinschlüssel is a PhD candidate in the Chair of Technical Electrochemistry Gasteiger research group at TUM. His current work encompasses an in-depth understanding of the complex interplay of cathode and electrolyte degradation mechanisms in lithium ion batteries using operando lab-based and synchrotron techniques. Leonhard received his MSc in chemistry from TUM, where, in his thesis for the Gasteiger group, he investigated the mitigation of aging of FeNC-based cathode catalyst layers in PEMFCs .
Learn more about upcoming ECS Webinars and review our previous webinar recordings.
Thanks to the webinar sponsors who make these complimentary programs possible!
 |
 |
 |
 |
Interested in presenting in the ECS Webinar Series? Email your presentation title and abstract to education@electrochem.org for consideration.