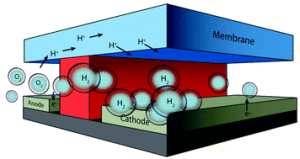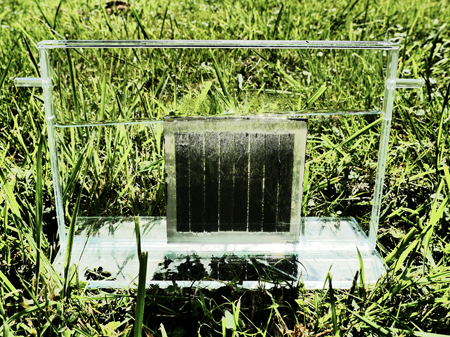
Image: Forschungszentrum Juelich
Artificial photosynthesis has carved out a promising corner in renewable energy research in recent history. This novel process is solar-driven, harvesting renewable energy and storing in in chemical bonds. Breakthroughs in artificial photosynthesis could lead to the development of solar fuels that could potentially shift the energy infrastructure.
However, while many technological barriers have been surpassed in the advancement of artificial photosynthesis, there are still hurdles to overcome. However, a research team from Forschungszentrum Juelich believes they may have just taken a significant step forward in the advancement of this field.
In a recently published paper, the team of scientists state that they have developed the first complete and compact design for an artificial photosynthesis facility.
The artificial photosynthesis process was first investigated in the 1970s. In fact, ECS Fellow Allen J. Bard can be seen here discussing the process in 1983. But only recently has artificial photosynthesis began to garner larger amounts of attention from the scientific community as a whole.



![[Click to enlarge]](https://www.electrochem.org/wp-content/uploads/2015/09/JCAP-T1_4-graphics-300x241.jpeg)