Our guest on this episode of the ECS Podcast is Shirley Meng, professor of NanoEngineering at the University of California, San Diego. Meng founded the Sustainable Power and Energy Center, the goal of which is solving key technical challenges in distributed energy generation, storage, and power management.
Meng is also the principal investigator of Laboratory for Energy Storage and Conversion research group. Her group is focused on functional nano and micro-scale materials for energy storage and conversion.
She talked to Rob Gerth, ECS’s director of marketing and communications.
Listen to the podcast and download this episode and others for free on Apple Podcasts, SoundCloud, Podbean, or our RSS Feed. You can also find us on Stitcher and Acast.


 A new kind of lithium sulfur battery could be more efficient, less expensive, and safer than currently available lithium batteries.
A new kind of lithium sulfur battery could be more efficient, less expensive, and safer than currently available lithium batteries. A new sodium-based battery can store the same amount of energy as a state-of-the-art lithium ion at a substantially lower cost.
A new sodium-based battery can store the same amount of energy as a state-of-the-art lithium ion at a substantially lower cost.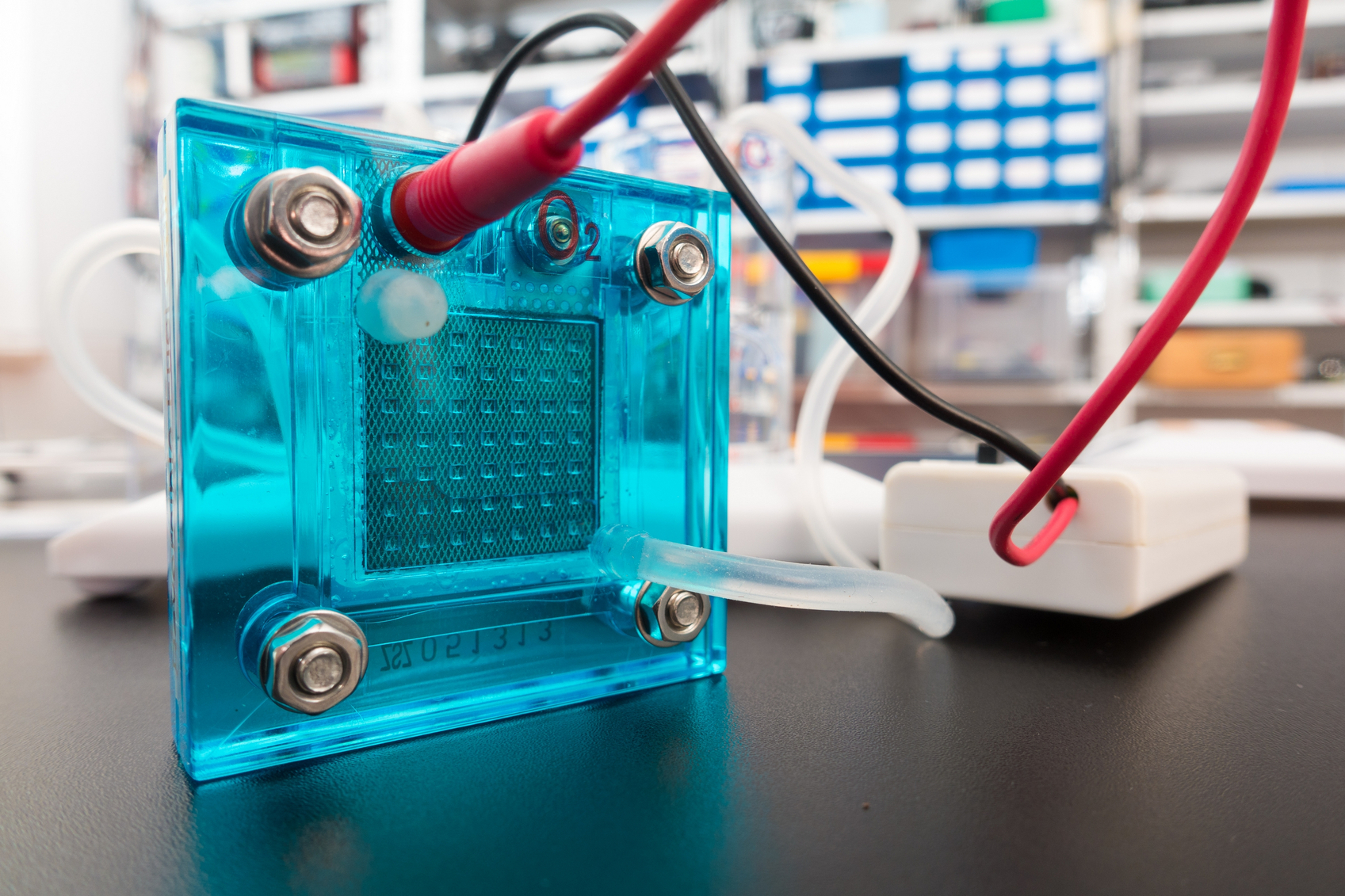 A closer look at catalysts is giving researchers a better sense of how these atom-thick materials produce hydrogen.
A closer look at catalysts is giving researchers a better sense of how these atom-thick materials produce hydrogen. Just a few months ago, business magnate Elon Musk announced that he would spearhead an effort to build the
Just a few months ago, business magnate Elon Musk announced that he would spearhead an effort to build the  Lithium batteries made with asphalt could charge 10 to 20 times faster than the commercial lithium-ion batteries currently available.
Lithium batteries made with asphalt could charge 10 to 20 times faster than the commercial lithium-ion batteries currently available.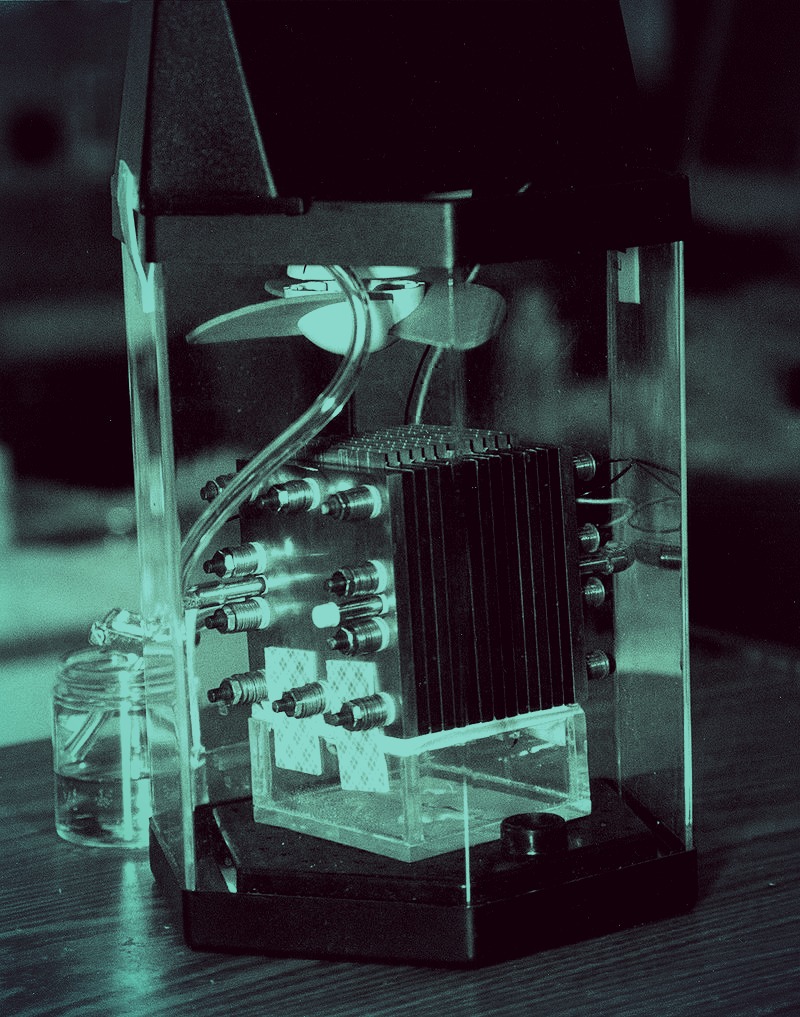
 The
The