 A team of researchers from the University of Toronto is looking to give wasted materials new value by developing a new catalyst that could help recycle carbon dioxide into plastic.
A team of researchers from the University of Toronto is looking to give wasted materials new value by developing a new catalyst that could help recycle carbon dioxide into plastic.
According to a new study, the researchers have successfully used a new technique to efficiently convert carbon dioxide to ethylene, which can then be processed to make polyethylene, the most common plastic used in making packaging, bottles, and toys.
By using a copper catalyst, the team was able to achieve the desired result of ethylene production. However, controlling the catalyst was one of the technological challenges the team had to overcome.


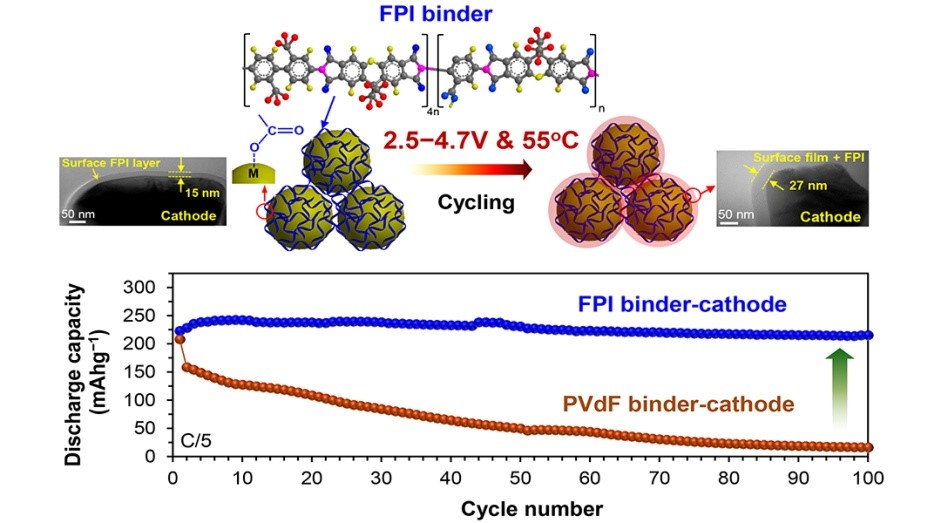
 One year ago, the Chinese government’s energy agency made a long-term commitment to the development of renewable energy sources, investing more than
One year ago, the Chinese government’s energy agency made a long-term commitment to the development of renewable energy sources, investing more than  Water-based rechargeable batteries could be one step closer to commercial viability, thanks to
Water-based rechargeable batteries could be one step closer to commercial viability, thanks to 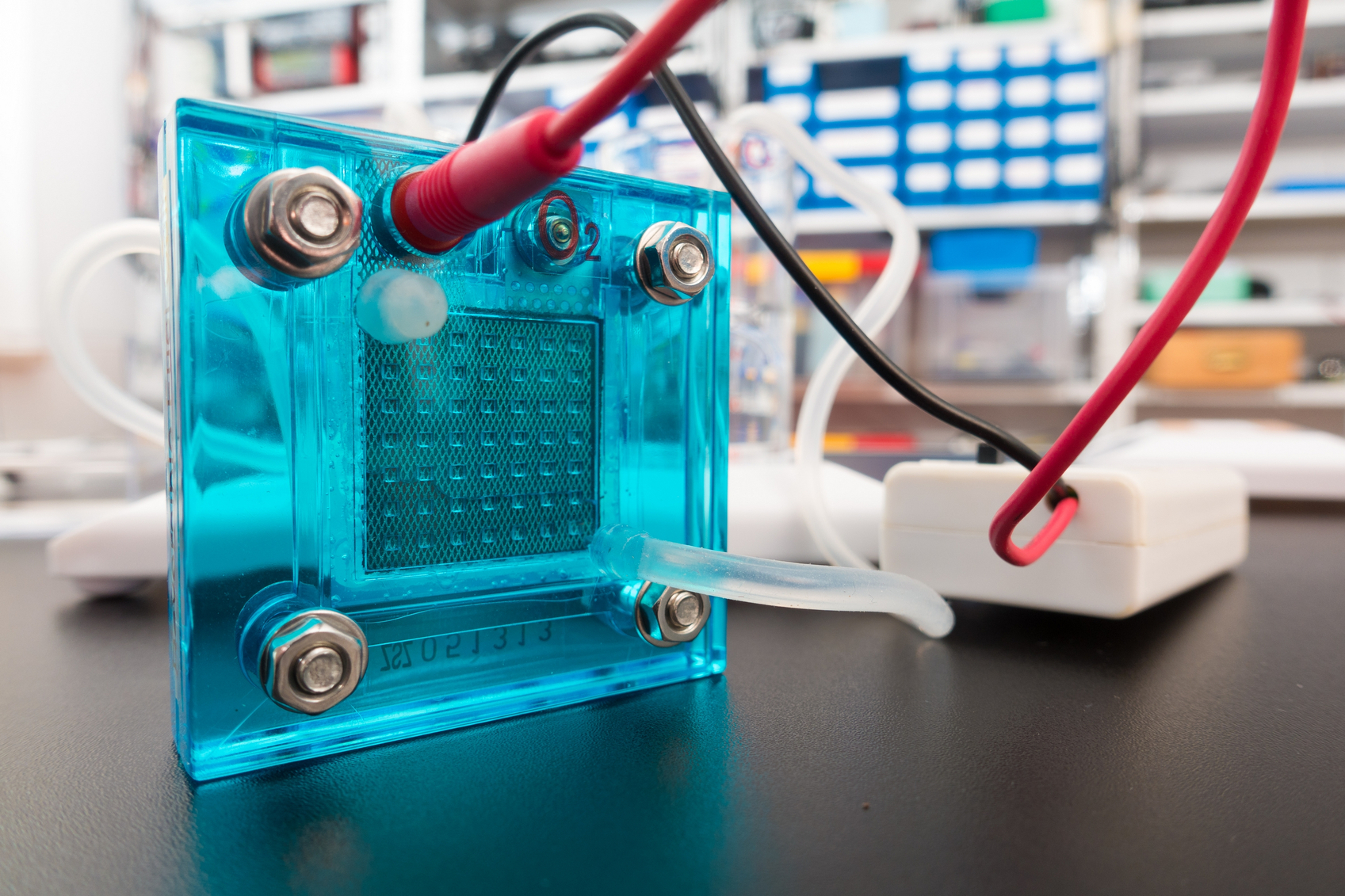 Nitrogen-doped carbon nanotubes or modified graphene nanoribbons could be effective, less costly replacements for expensive platinum in fuel cells, according to a new study.
Nitrogen-doped carbon nanotubes or modified graphene nanoribbons could be effective, less costly replacements for expensive platinum in fuel cells, according to a new study.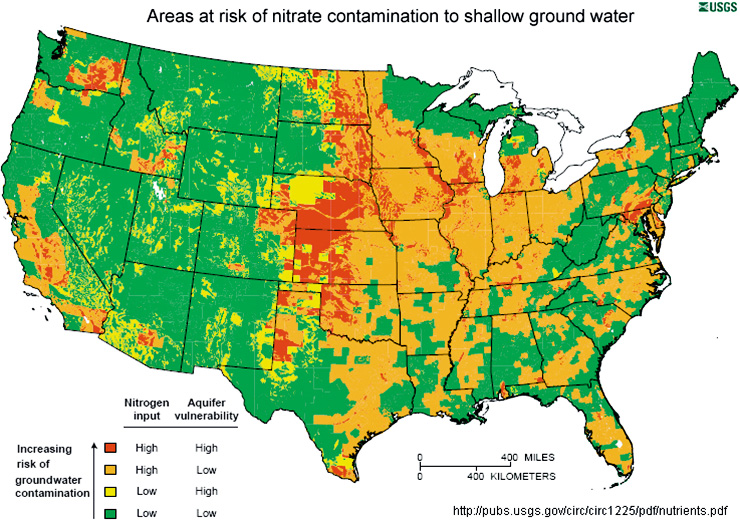
 ECS members
ECS members  New research from Sandia National Laboratory is moving toward advancing solid state lithium-ion battery performance in small electronics by identifying major obstacles in how lithium ions flow across battery interfaces.
New research from Sandia National Laboratory is moving toward advancing solid state lithium-ion battery performance in small electronics by identifying major obstacles in how lithium ions flow across battery interfaces.