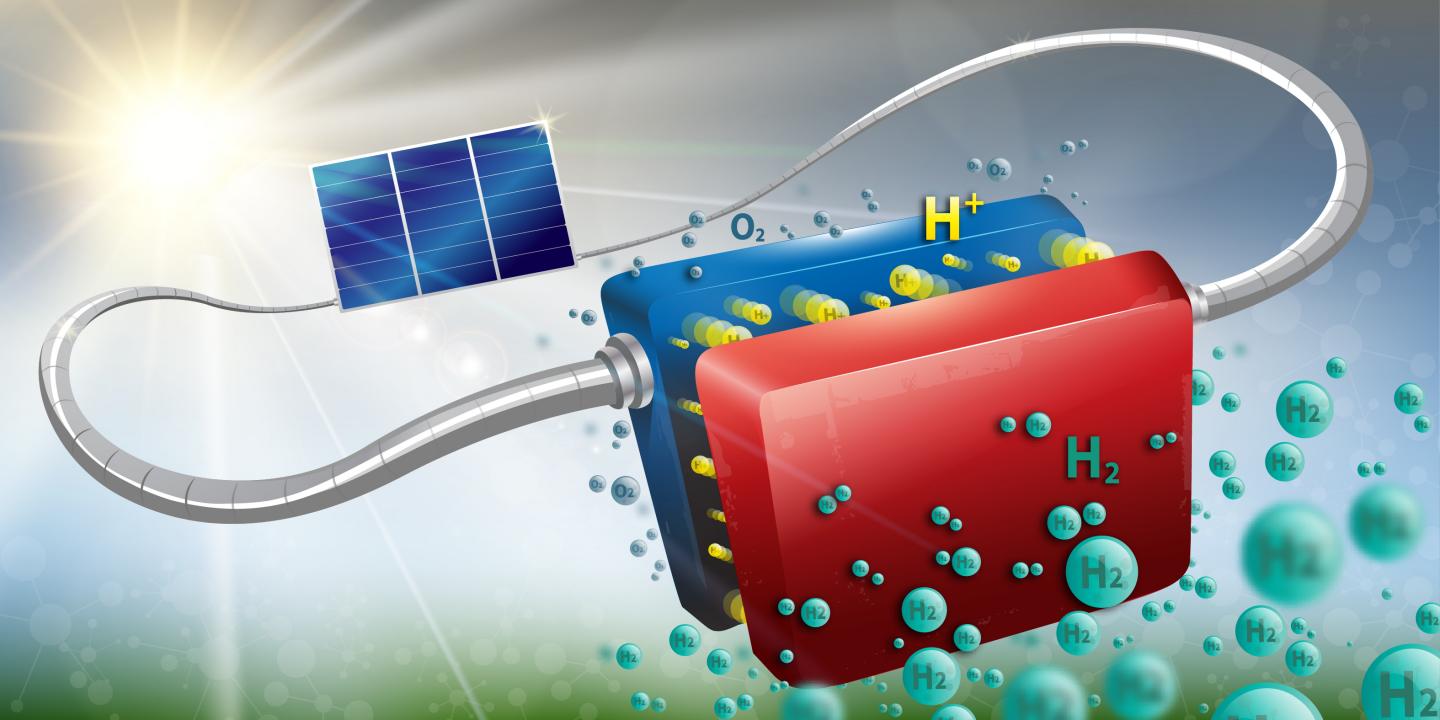
The device is able to convert solar energy into hydrogen at a rate of 14.2 percent, and has already been run for more than 100 hours straight.
Image: Infini Lab/EPFL
One of the biggest barriers between renewables and widespread grid implementation has been the issue of intermittency. How can we meet a nation’s energy demands with solar when the sun goes down?
In an effort to move past these barriers toward a cleaner energy infrastructure, a new paper published in the Journal of The Electrochemical Society describes an effective, low-cost solution for storing solar energy.
The research team from Ecole Polytechnique Fédérale de Lausanne is looking to covert solar energy into hydrogen through water electrolysis. At its core, the concept revolves around using solar-produced electricity to split water molecules into hydrogen and oxygen, leaving clean hydrogen to be stored as future energy or even as a fuel.
But this idea is not new to the scientific community. However, the research published in JES provides answer to continuous barriers in this field related to stability, scaling, and efficiency.


 The world’s next energy revolution is looming nearer.
The world’s next energy revolution is looming nearer.
 Electric vehicles have become more visible in the automobile market over the past few years, but many potential buyers still cite one thing as a major deterrent in going electric: range anxiety.
Electric vehicles have become more visible in the automobile market over the past few years, but many potential buyers still cite one thing as a major deterrent in going electric: range anxiety. Many scientists believe we’re at the tipping point of our energy technology future. With the advancement of new, alternative energy sources, some are left to wonder what will happen to the energy landscape as a whole.
Many scientists believe we’re at the tipping point of our energy technology future. With the advancement of new, alternative energy sources, some are left to wonder what will happen to the energy landscape as a whole. A team of researchers from the National Renewable Energy Laboratory, in collaboration with a team from Shanghai Jiao Tong University, has developed a method to improve perovskite solar cells – raising both efficiency and reliability levels while make them easier to produce.
A team of researchers from the National Renewable Energy Laboratory, in collaboration with a team from Shanghai Jiao Tong University, has developed a method to improve perovskite solar cells – raising both efficiency and reliability levels while make them easier to produce. Researchers have taken a step toward the development of renewable plastics – a promising transformation from current plastics made from oil. The biodegradable material is possible due to the creation of a new catalyst.
Researchers have taken a step toward the development of renewable plastics – a promising transformation from current plastics made from oil. The biodegradable material is possible due to the creation of a new catalyst.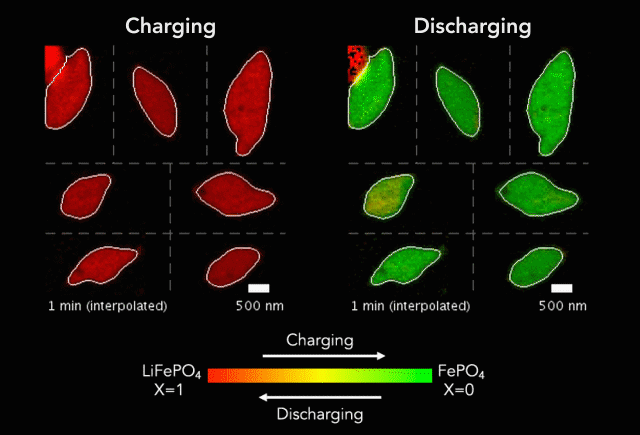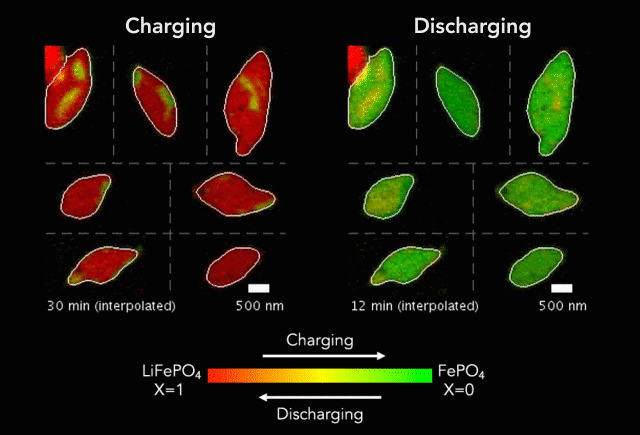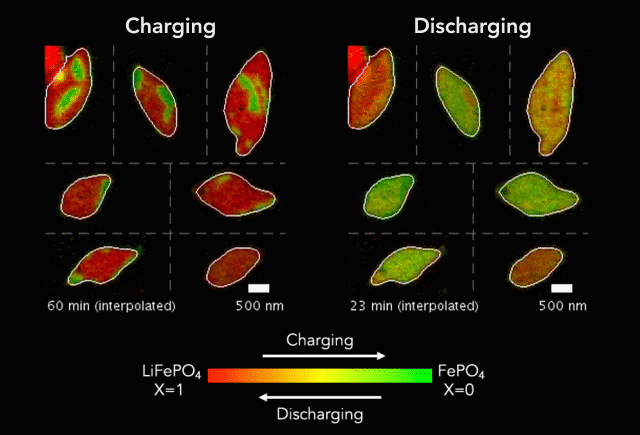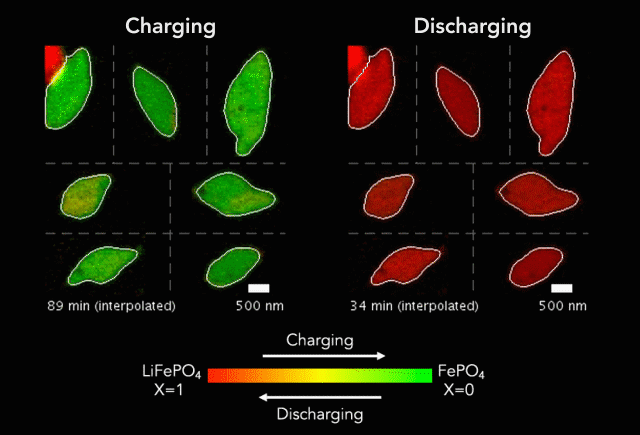
 A
A