By: Mark Barteau, University of Michigan
 President…Donald…Trump. For those on both sides of the aisle who vowed “Never Trump!,” that’s going to take some getting used to. On this morning after a stunning election, the first impulse may be to describe the future in apocalyptic phrases. Game over for the climate! Game over for NATO! Game over for the Clean Power Plan! Game over for Planned Parenthood!
President…Donald…Trump. For those on both sides of the aisle who vowed “Never Trump!,” that’s going to take some getting used to. On this morning after a stunning election, the first impulse may be to describe the future in apocalyptic phrases. Game over for the climate! Game over for NATO! Game over for the Clean Power Plan! Game over for Planned Parenthood!
While there are certainly extreme outcomes possible for these and many other issues that divide our nation, we may see some moderation, especially on matters where the divisions do not rigidly follow ideological fault lines.
Of course, the president-elect himself is famous neither for hewing to right wing orthodoxy nor for consistency between his various pronouncements. As he has said: “I like to be unpredictable.”
But make no mistake, in the energy and climate space Trump’s number one priority is to dismantle the Obama legacy as he sees it. And he sees it largely through the lens of organizations like the U.S. Chamber of Commerce and the American Petroleum Institute, pro-fossil fuel organizations severely allergic to regulations.
A prime target is the Environmental Protection Agency and its regulation of greenhouse gases via the Clean Power Plan and methane emissions measures, which are described as “job killers.”
Fossil fuel revolution
The Clean Power Plan, which sets limits on carbon emissions from power plants, has been stayed by the courts for the moment, but one should not forget that EPA’s responsibility to regulate CO2 emissions under the Clean Air Act was affirmed by the Supreme Court. This sets up a potential conflict among the executive, legislative and judicial branches.
President Trump and a Republican-controlled Congress may hollow out and handcuff the EPA, but EPA’s responsibility to regulate greenhouse gases will remain unless existing law is modified by Congress or by a Court returned to full strength with Trump appointees.
(more…)
 Renewable energy efforts around the world have grown exponentially over the past few years. Countries such as Japan have developed the world’s largest floating solar project, initiatives like Solar Hope are working to provide clean energy to sub-Saharan Africa, and Hawaii is leading the charge in the U.S. with its commitment to 100 percent clean energy by 2045. Now, the Netherlands has marked a new milestone in renewables by implementing a total of 2,200 wind turbines.
Renewable energy efforts around the world have grown exponentially over the past few years. Countries such as Japan have developed the world’s largest floating solar project, initiatives like Solar Hope are working to provide clean energy to sub-Saharan Africa, and Hawaii is leading the charge in the U.S. with its commitment to 100 percent clean energy by 2045. Now, the Netherlands has marked a new milestone in renewables by implementing a total of 2,200 wind turbines.

 President…Donald…Trump. For those on both sides of the aisle who vowed “Never Trump!,” that’s going to take some getting used to. On this morning after a stunning election, the first impulse may be to describe the future in apocalyptic phrases. Game over for the climate! Game over for NATO! Game over for the Clean Power Plan! Game over for Planned Parenthood!
President…Donald…Trump. For those on both sides of the aisle who vowed “Never Trump!,” that’s going to take some getting used to. On this morning after a stunning election, the first impulse may be to describe the future in apocalyptic phrases. Game over for the climate! Game over for NATO! Game over for the Clean Power Plan! Game over for Planned Parenthood!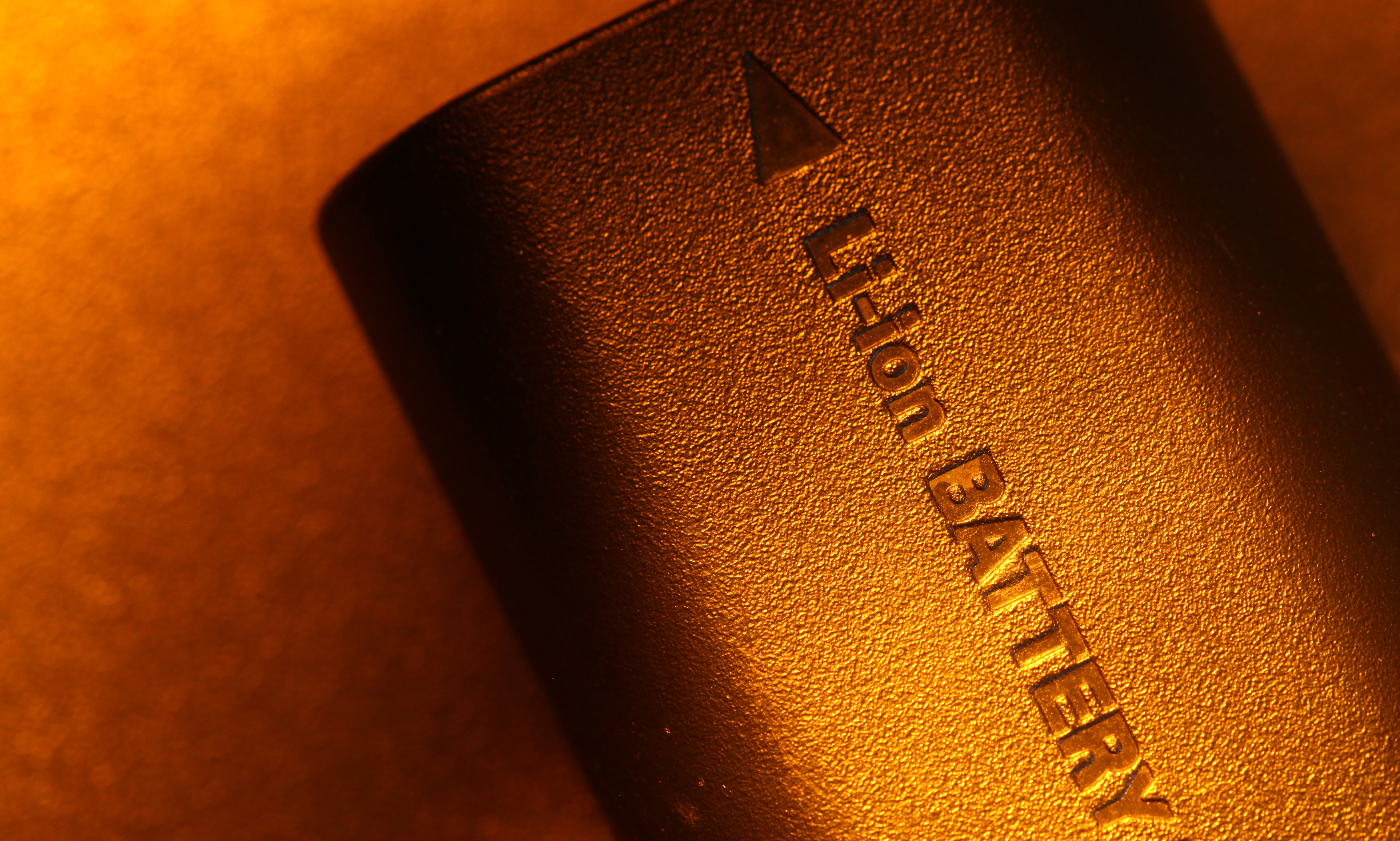
 In 2005, the number of electric vehicles on the road could be measured in the hundreds. Over the years, researchers have made technological leaps in the field of EVs. Now, we’ve exceeded a global threshold of
In 2005, the number of electric vehicles on the road could be measured in the hundreds. Over the years, researchers have made technological leaps in the field of EVs. Now, we’ve exceeded a global threshold of 
 Lithium-air batteries are viewed by many as a potential next-generation technology in energy storage. With the highest theoretical energy density of all battery devices, Li-air could revolutionize everything from electric vehicles to large-scale grid storage. However, the relatively young technology has a few barriers to overcome before it can be applied. A new study published in the
Lithium-air batteries are viewed by many as a potential next-generation technology in energy storage. With the highest theoretical energy density of all battery devices, Li-air could revolutionize everything from electric vehicles to large-scale grid storage. However, the relatively young technology has a few barriers to overcome before it can be applied. A new study published in the