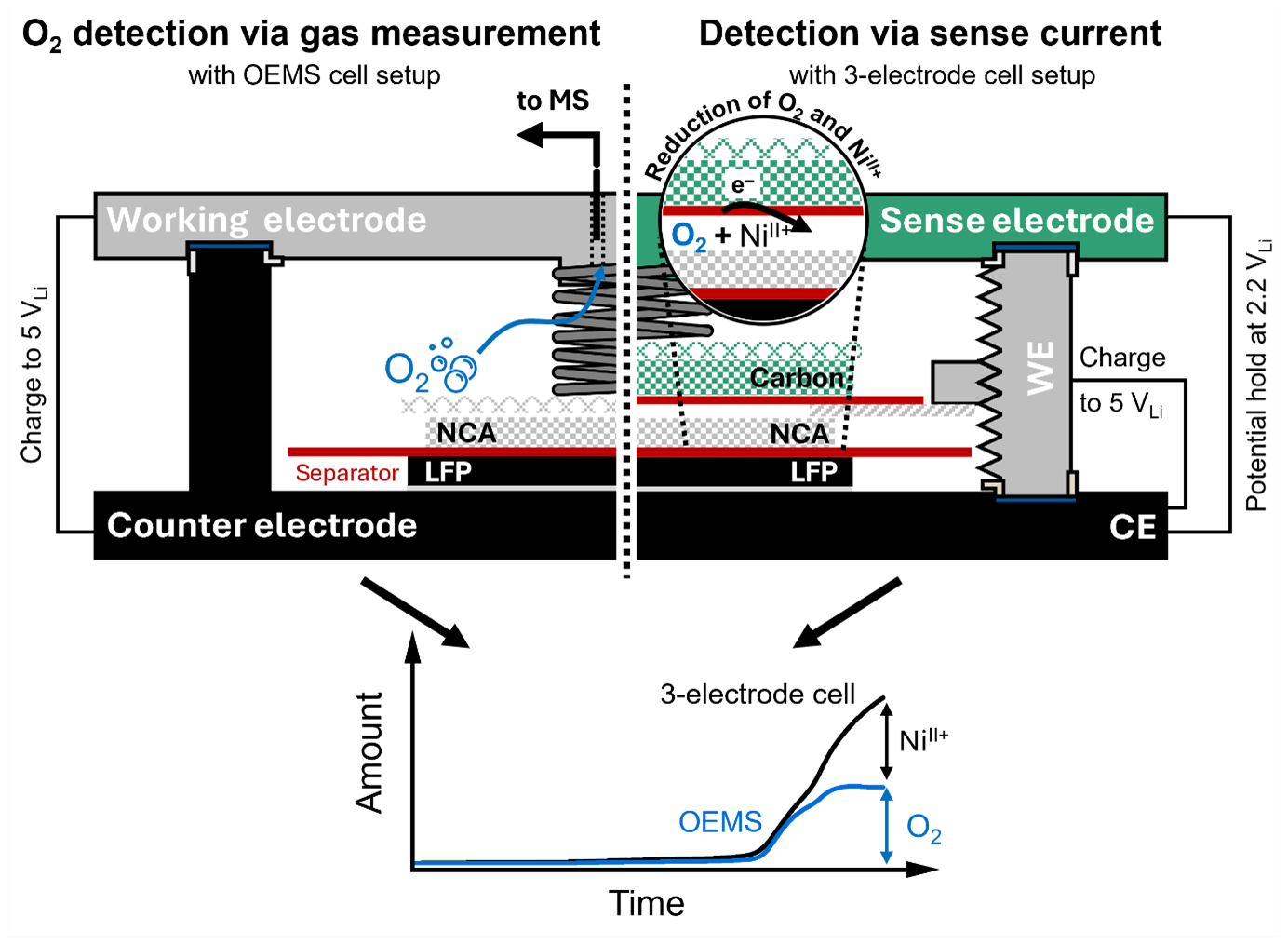
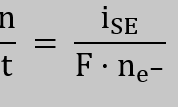The Electrochemical Society hosted “Development and Application of a 3-Electrode Setup for the Operando Detection of Side Reactions in Li-Ion Batteries,” a live webinar by Lennart Reuter and Leonhard J. Reinschlüssel (Technische Universität München), on August 6, 2025. A live Question and Answer session followed. Answers to some of the questions not addressed during the broadcast follow.
Registration is required to view the webinar replay.
Q&A
What was the reason to use a delithiated LFP as a CE in the 3-electrode cell?
This is an excellent question. As for the choice of counter electrode (CE) in the 3-electrode cell, one needs to consider a variety of factors:
-
To delithiate a cathode active material (CAM), or polarize a carbon electrode, the CE must have sufficient capacity for (de‑)lithiation. That is, it must be capacitively oversized relative to the used CAM loading, or pre‑lithiated for polarization of a carbon electrode.
-
The CE must sit at a constant potential over a wide range of state of charge (SOC) in order to accurately define both the potential of the WE during its polarization, as well as the constant potential at which the SE is being held throughout the measurement.
-
The CE must be electrochemically “inert,” meaning that no species are being built or reduced within its operation voltage. Therefore, the potential of the CE must sit high enough to not reduce (or low enough to not oxidize) formed species, as for example O2 or TM‑ions, which have reduction potentials between 2.2 – 2.8 VLi.
As the operation voltages of graphite, lithium metal, and lithiated LTO are either not constant over a wide SOC window, and/or well below the reduction potential of most of the species of interest, a delithiated LFP CE with a flat and stable potential on ≈ 3.42 VLi fulfills all of the requirements stated above.
A huge challenge associated with using a sensing electrode is their selectivity. How flexible is the setup with regards to change of the electrolyte or electrode materials?
This is indeed a critical point on which we would like to further elaborate.
Especially when investigating parasitic side reactions in LIBs, many of them are either a result of each other or occur within a narrow potential window, making their deconvolution difficult. The use of a sense electrode does not get rid of this problem, as the detection of species via their reduction or oxidation at the SE depends highly on the reduction potential of the species of interest. Especially when using a carbon SE, the reduction potentials of species such as O2 or TM‑ions are at least overlapping to a certain degree, making their respective deconvolution, if sensed at the same time, difficult. However, by sensible choice of SE potential, and comparison thereof, a mechanistic deconvolution is still possible (e.g., as shown in our talk for the example of O2‑release versus TM dissolution).
Beyond that, the selectivity of the SE towards a single species can be improved by choosing adequate SE materials. In this regard, we mainly focused on the reduction of H+ (either free protons and/or as HF) in our talk, in which case the selectivity can be improved by using a platinum containing SE (due to the excellent HER activity of Pt/C). If one would aim to further improve the selectivity towards the detection of, for example O2, changing to a Pd/C as an excellent catalyst for the ORR could be one approach.
Beyond optimization/tailoring of the SE material, the 3‑electrode cell setup is highly flexible toward material screening. No limits in terms of electrolyte usage—beyond those in an actual LIB—are known to us and a variety of conduction salt/solvent compositions have already been screened. Likewise, all electrode materials spanning from layered transition metal oxides to olivines and high voltage spinels.
Have you also applied analytical practices to show the detection limit for these different species?
Yes, we did. The detection limit is determined by how precisely the current response of the SE can be measured once a reductive species is monitored. The precision of the experiment depends on the noise () of the current signal, from which the limit of detection () and the limit of quantification () can be derived (for details, see T. O’Haver, “Pragmatic Introduction to Signal Processing: Applications in Scientific Measurement,” [2022]).
We determined the noise of the SE current as its standard, during the initial 4 h OCV period of the NCA working electrode, which was 60 nA. At an NCA loading of 10 mgNCA/cm2 and an electrode area of 1.72 cm2, this corresponds to a specific current of . This now allows us to convert the LOQ for O2 (2.3 e–/O2 molecule) and TM detection (2 e–/TM+II ion) considering Faraday’s law:
Have you also applied analytical practices to show the detection limit for these different species?
Yes, we did. The detection limit is determined by how precisely the current response of the SE can be measured once a reductive species is monitored. The precision of the experiment depends on the noise () of the current signal, from which the limit of detection () and the limit of quantification () can be derived (for details, see T. O’Haver, “Pragmatic Introduction to Signal Processing: Applications in Scientific Measurement,” [2022]).
We determined the noise of the SE current as its standard, during the initial 4 h OCV period of the NCA working electrode, which was 60 nA. At an NCA loading of 10 mgNCA/cm2 and an electrode area of 1.72 cm2, this corresponds to a specific current of . This now allows us to convert the LOQ for O2 (2.3 e–/O2 molecule) and TM detection (2 e–/TM+II ion) considering Faraday’s law:
Considering the number of electrons involved in the reduction, we obtain the following values for the limit of quantification for O2 and TM as following:
How do you take into account the polarization of counter electrode?
Thank you for this excellent question. It points in the right direction, namely that the potential of the LFP CE changes once it is polarized. To estimate the extent of this polarization, we can refer to impedance data for LFP electrodes provided in the following study: S. Solchenbach et al., “A Gold Micro-Reference Electrode for Impedance and Potential Measurements in Lithium-Ion Batteries,” Journal of The Electrochemical Society, 163, A2265–A2272 (2016). In this work, an LFP electrode with a similar loading and temperature was analysed, and the charge-transfer resistance was determined to be ~2 Ω cm2.
If we polarize an LFP electrode with a loading of 22 mgLFP/cm2 at a specific current of 33 mA/gLFP (corresponding to C/5), the resulting voltage drop across the electrode would be ~1.5 mV. Consequently, when the LFP CE electrode is polarized, the potential of the SE would shift by the same ~1.5 mV toward lower potentials. We considered this effect to be negligible in sensing reductive species.
Publications
J. Reinschlüssel, L. Reuter, P. Rapp, M. Bock, A. Berger, M. A. Schilling and H. Gasteiger, “Temperature Dependence of the Chemical vs. the Electrochemical Electrolyte Oxidation in Lithium-Ion Batteries with Ni rich CAMs: Gas Evolution, Salt Decomposition, and Metal Dissolution” Journal of The Electrochemical Society, 172, 060536 (2025) https://doi.org/10.1149/1945-7111/ade0ec.
Reuter, L. J. Reinschlüssel and H. A. Gasteiger, “3-Electrode Setup for the Operando Detection of Side Reactions in Li-Ion Batteries: The Quantification of Released Lattice Oxygen and Transition Metal Ions from NCA” Journal of The Electrochemical Society, 171, 100524 (2024).
Learn more about upcoming ECS Webinars and review our previous webinar recordings.
Thanks to the webinar sponsors who make these complimentary programs possible!
 |
 |
 |
 |
Interested in presenting in the ECS Webinar Series? Email your presentation title and abstract to education@electrochem.org for consideration.