 The U.S. Department of Energy (DOE) released a report Wednesday night on electricity markets and grid reliability, stating that the decline in coal and nuclear production has not impacted grid reliability, instead the rise in a diverse energy portfolio has increased the grid’s stability.
The U.S. Department of Energy (DOE) released a report Wednesday night on electricity markets and grid reliability, stating that the decline in coal and nuclear production has not impacted grid reliability, instead the rise in a diverse energy portfolio has increased the grid’s stability.
The study, commissioned by Energy Secretary Rick Perry in April, also states that coal plant closures across the country have been due to market pressure and competition from low-priced natural gas plants, not policy changes that support renewables such as wind and solar.
(MORE: Listen to our interview with former U.S. Energy Secretary and Nobel Laureate Steven Chu.)
“America is also fortunate to have a variety of fuel sources. We need to consider how to use each effectively while recognizing our differences and unique state and regional circumstances,” Perry says in the report’s cover letter. “We must utilize the most effective combination of energy sources with an ‘all of the above’ approach to achieve long-term, reliable American energy security.”
While the report does not state that there is a current concern with grid reliability, it does warn that future problems could arise if coal and nuclear plants continue to close at the current rate. Many environmental advocates cite this as a last-ditch effort for these companies to remain relevant in the energy landscape. However, the report does go on to highlight the role of renewables in developing a diverse energy infrastructure.


 Researchers have created a new method to more efficiently convert potato waste into ethanol. The findings may lead to reduced production costs for biofuel in the future and add extra value for chip makers.
Researchers have created a new method to more efficiently convert potato waste into ethanol. The findings may lead to reduced production costs for biofuel in the future and add extra value for chip makers.
 While pursing work on the highly desirable but technically challenging lithium-air battery, researchers unexpectedly discovered a new way to capture and store carbon dioxide. Upon creating a design for a lithium-CO2 battery, the research team found a way to isolate solid carbon dust from gaseous carbon dioxide, all while being able to separate oxygen.
While pursing work on the highly desirable but technically challenging lithium-air battery, researchers unexpectedly discovered a new way to capture and store carbon dioxide. Upon creating a design for a lithium-CO2 battery, the research team found a way to isolate solid carbon dust from gaseous carbon dioxide, all while being able to separate oxygen. This summer I worked on the Greenland ice sheet, part of a scientific experiment to study surface melting and its contribution to Greenland’s accelerating ice losses. By virtue of its size, elevation and currently frozen state, Greenland has the potential to cause large and rapid increases to sea level as it melts.
This summer I worked on the Greenland ice sheet, part of a scientific experiment to study surface melting and its contribution to Greenland’s accelerating ice losses. By virtue of its size, elevation and currently frozen state, Greenland has the potential to cause large and rapid increases to sea level as it melts. Researchers have found a new method for finding lithium, used in the lithium-ion batteries that power modern electronics, in supervolcanic lake deposits.
Researchers have found a new method for finding lithium, used in the lithium-ion batteries that power modern electronics, in supervolcanic lake deposits.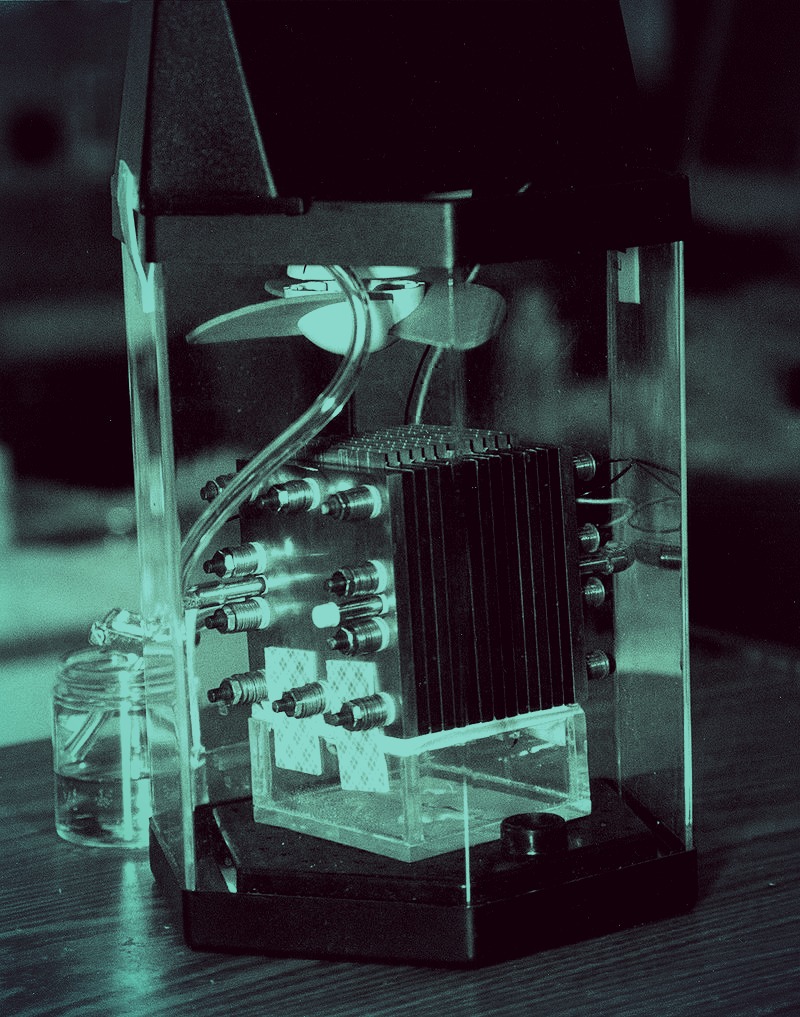

 Researchers from Lappeenranta University of Technology (LUT) and VTT Technical Research Centre of Finland have successfully created food out of electricity and carbon dioxide, which they hope could one day be used to help solve world hunger.
Researchers from Lappeenranta University of Technology (LUT) and VTT Technical Research Centre of Finland have successfully created food out of electricity and carbon dioxide, which they hope could one day be used to help solve world hunger.