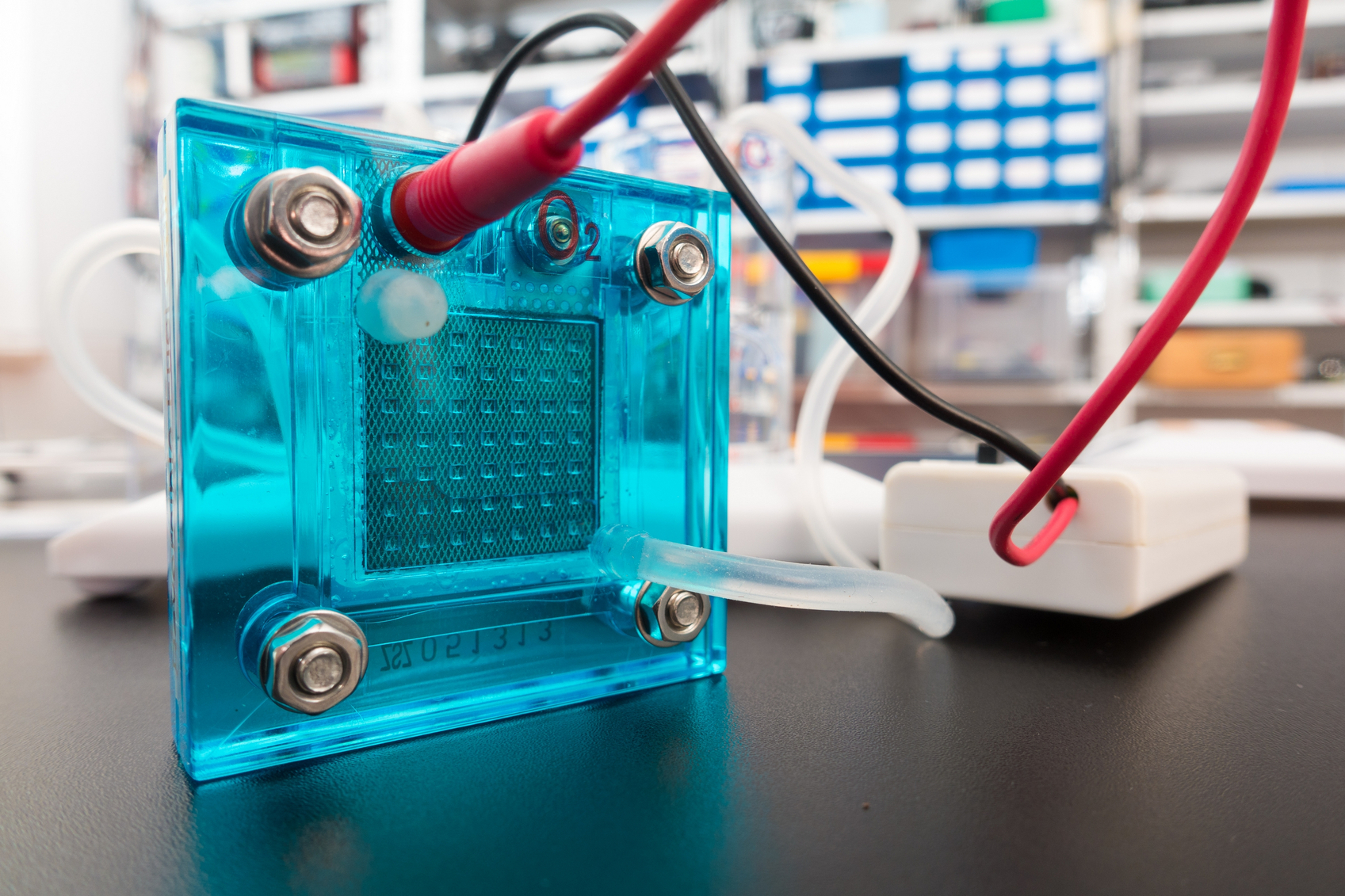
 A closer look at catalysts is giving researchers a better sense of how these atom-thick materials produce hydrogen.
A closer look at catalysts is giving researchers a better sense of how these atom-thick materials produce hydrogen.
Their findings could accelerate the development of 2D materials for energy applications, such as fuel cells.
The researchers’ technique allows them to probe through tiny “windows” created by an electron beam and measure the catalytic activity of molybdenum disulfide, a two-dimensional material that shows promise for applications that use electrocatalysis to extract hydrogen from water.
Initial tests on two variations of the material proved that most production is coming from the thin sheets’ edges.
Researchers already knew the edges of 2D materials are where the catalytic action is, so any information that helps maximize it is valuable, says Jun Lou, a professor of materials science and nanoengineering at Rice University whose lab developed the technique with colleagues at Los Alamos National Laboratory.


 Engineers working to make solar cells more cost effective ended up finding a method for making sonar-like collision avoidance systems in self-driving cars.
Engineers working to make solar cells more cost effective ended up finding a method for making sonar-like collision avoidance systems in self-driving cars. Just a few months ago, business magnate Elon Musk announced that he would spearhead an effort to build the
Just a few months ago, business magnate Elon Musk announced that he would spearhead an effort to build the  Lithium batteries made with asphalt could charge 10 to 20 times faster than the commercial lithium-ion batteries currently available.
Lithium batteries made with asphalt could charge 10 to 20 times faster than the commercial lithium-ion batteries currently available.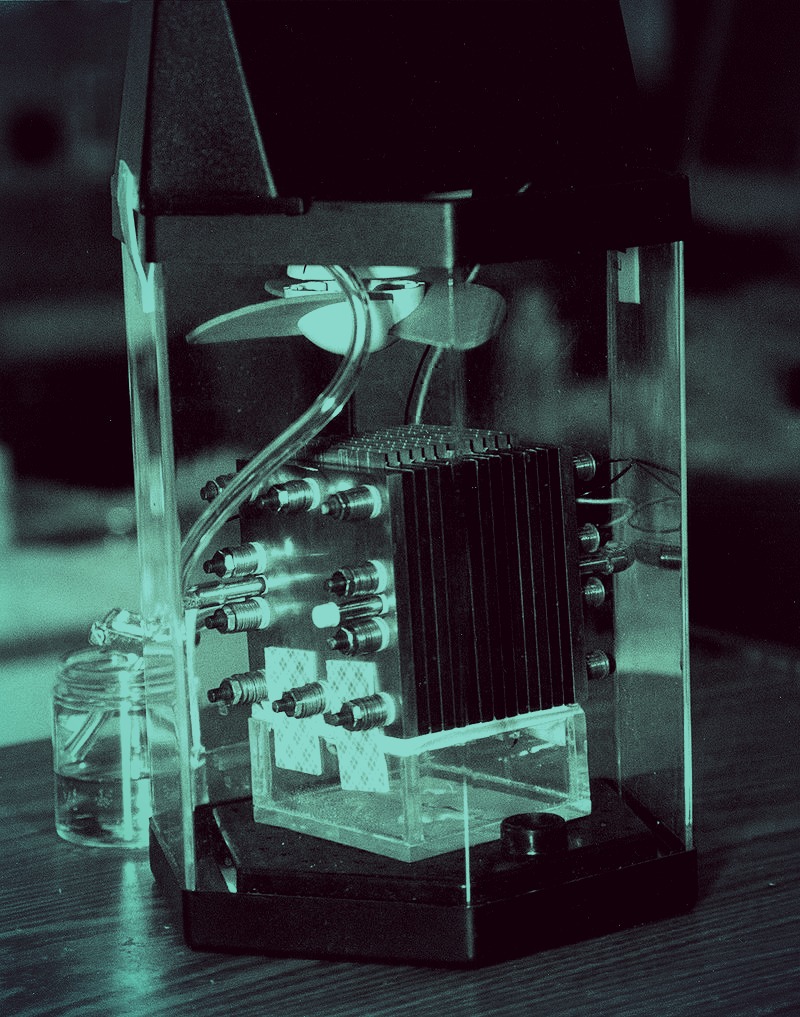
 A novel compound called 3Q conducts electricity and retains energy better than other organic materials currently used in batteries, researchers report.
A novel compound called 3Q conducts electricity and retains energy better than other organic materials currently used in batteries, researchers report. The National Science Foundation is spearheading a $2.4 million research initiative to develop new methods to create commercial fertilizer out of wastewater nutrients. Among the researchers working on this project, ECS member and chair of the Society’s Energy Technology Divison, Andrew Herring, is leading an electrochemical engineering team in electrode design, water chemistry, electrochemical operations, and developing a bench-scale electrochemical reactor design.
The National Science Foundation is spearheading a $2.4 million research initiative to develop new methods to create commercial fertilizer out of wastewater nutrients. Among the researchers working on this project, ECS member and chair of the Society’s Energy Technology Divison, Andrew Herring, is leading an electrochemical engineering team in electrode design, water chemistry, electrochemical operations, and developing a bench-scale electrochemical reactor design. In a
In a