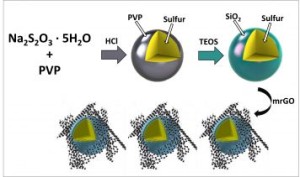
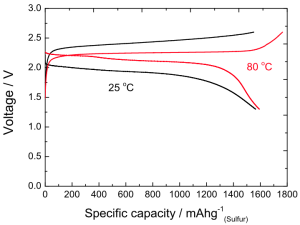
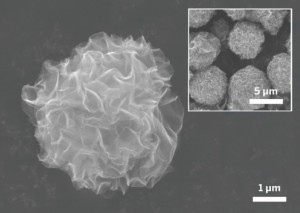
Ningde Amperex Technology Ltd. (ATL, China) is announcing a funding opportunity for researchers actively engaged in rechargeable lithium battery technologies. They are offering $100,000-$500,000 to selected projects addressing current problems associated with lithium metal anodes and proposing viable solutions for the commercialization of long-life, high-performance lithium metal secondary batteries for high energy density applications.
Ningde Amperex Technology Ltd. (ATL, China) is announcing a funding opportunity for researchers actively engaged in rechargeable lithium battery technologies. They are offering $100,000-$500,000 to selected projects addressing current problems associated with lithium metal anodes and proposing viable solutions for the commercialization of long-life, high-performance lithium metal secondary batteries for high energy density applications.
The steep demand for improved rechargeable batteries for use in consumer electronics and electric vehicles is driving the search for new battery electrode materials that will achieve higher energy densities. This funding opportunity seeks to develop scalable technologies for improving the performance of lithium metal anodes.
Please submit technical proposals along with a budget justification, confidentiality disclaimer and a cover page identifying the principle investigator, contact information, affiliations, project duration, total funding requested and submission date to Dr. KaiFu Zhong.
The deadline for submissions is July 31, 2015.