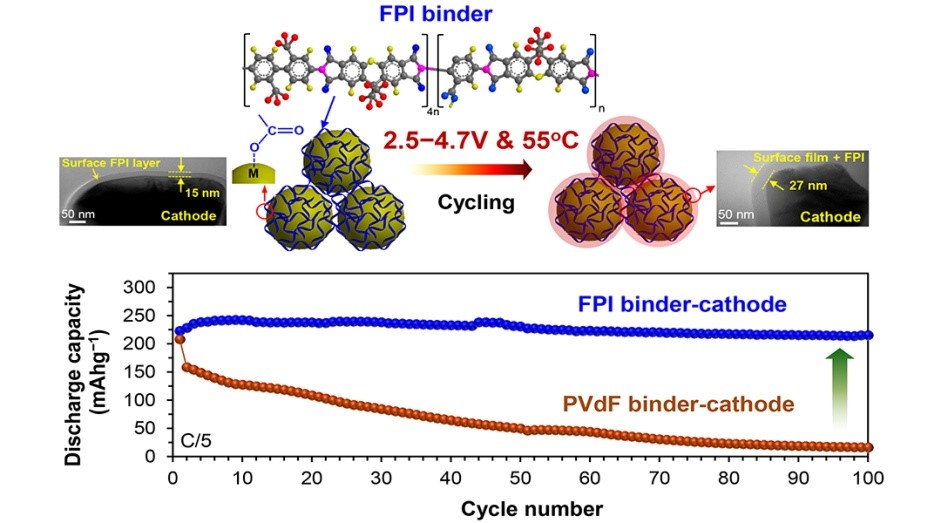
Superior high-voltage performance of Li-ion full cell with Li-rich layered oxide cathode prepared with fluorinated polyimide (FPI) binder, compared to the cell with conventional binder PVdF. (Click to enlarge.)
Image: Seung Wan Song
In order to increase the driving range of electric vehicles, researchers across the globe are working to develop lithium-ion batteries with higher energy storage. Now, scientists at Chungnam National University and Kumoh National Institute of Technology in Korea are taking a step toward that goal with their development of the first high-voltage cathode binder for higher energy Li-ion batteries.
Today’s Li-ion batteries are limited to charge to 4.2V due to the electrochemical instability of the liquid electrolyte and cathode-electrolyte interface, and loosening of conventional binder, polyvinylidenefluoride (PVdF), particularly at elevated temperatures. The fabrication of Li-rich layered oxide cathode with a novel high-voltage binder, as the research team demonstrated, can overcome these limitations.
Charging the batteries with Li-rich layered oxide cathode (xLi2MnO3∙(1−x)LiMO2, M = Mn, Ni, Co) to higher than 4.5V produces approximately doubled capacity than those with LiCoO2 cathode, so that doubled energy density batteries can be achieved.


 One year ago, the Chinese government’s energy agency made a long-term commitment to the development of renewable energy sources, investing more than
One year ago, the Chinese government’s energy agency made a long-term commitment to the development of renewable energy sources, investing more than  Water-based rechargeable batteries could be one step closer to commercial viability, thanks to
Water-based rechargeable batteries could be one step closer to commercial viability, thanks to  Nitrogen-doped carbon nanotubes or modified graphene nanoribbons could be effective, less costly replacements for expensive platinum in fuel cells, according to a new study.
Nitrogen-doped carbon nanotubes or modified graphene nanoribbons could be effective, less costly replacements for expensive platinum in fuel cells, according to a new study.
 ECS members
ECS members  New research from Sandia National Laboratory is moving toward advancing solid state lithium-ion battery performance in small electronics by identifying major obstacles in how lithium ions flow across battery interfaces.
New research from Sandia National Laboratory is moving toward advancing solid state lithium-ion battery performance in small electronics by identifying major obstacles in how lithium ions flow across battery interfaces. Carbon dioxide accounts for over
Carbon dioxide accounts for over 