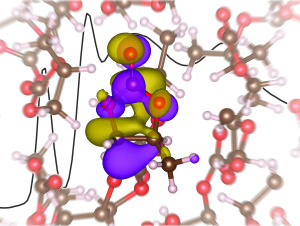
X-ray absorption spectra, interpreted using first-principles electronic structure calculations, provide insight into the solvation of the lithium ion in propylene carbonate.
Image: Rich Saykally, Berkeley Labs
The Electrochemical Society’s Stephen Harris, along with a team of researchers from Berkeley Lab, have found a possible avenue to a better electrolyte for lithium-ion batteries.
Harris – an expert on lithium-ion batteries and chemist at Berkeley Lab’s Materials Science Division – believes that he and his team have unveiled something that could lead to applying lithium-ion batteries to large-scale energy storage.
Researchers around the world know that in order for lithium-ion batteries to store electrical energy for the gird or power electric cars, they must be improved. The team at Berkeley decided to take on this challenge and found surprising results in the first X-ray absorption spectroscopy study of a model lithium electrode, which has provided a better understanding of the liquid electrolyte.
Previous simulations have predicted a tetrahedral solvation structure for the lithium-ion electrolyte, but the new study yields different results.