 The Electrochemical Society hosted Matthew D. L. Garayt’s live webinar, “A Comprehensive Method for Assembly & Design Optimization of Single-Layer Pouch Cells,” on October 23, 2024. A live Question and Answer session followed. Matthew’s answers to some of the questions not addressed during the broadcast follow.
The Electrochemical Society hosted Matthew D. L. Garayt’s live webinar, “A Comprehensive Method for Assembly & Design Optimization of Single-Layer Pouch Cells,” on October 23, 2024. A live Question and Answer session followed. Matthew’s answers to some of the questions not addressed during the broadcast follow.
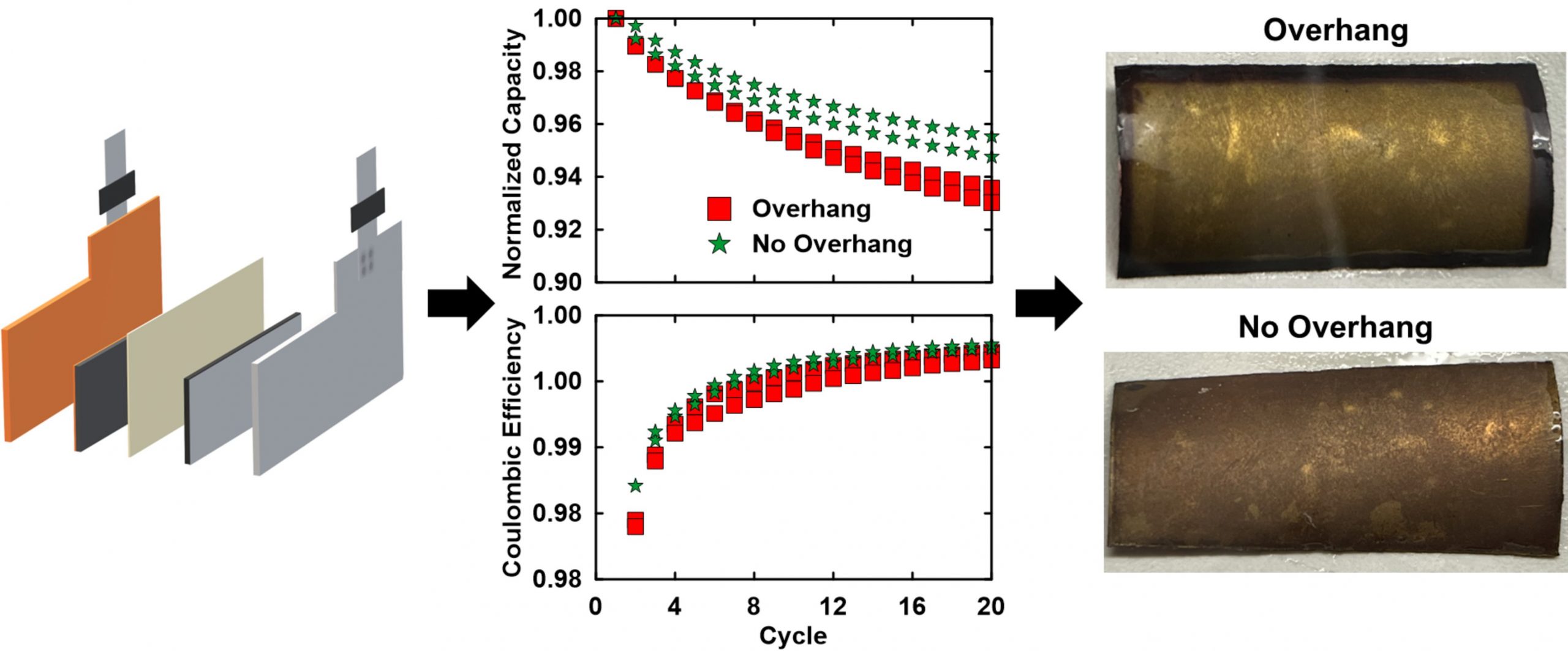
NOTE: Many of the questions submitted are addressed in “A Guide to Making Highly Reproducible Li-Ion Single-Layer Pouch Cells for Academic Researchers,” the paper Matthew and his colleagues published in the Journal of The Electrochemical Society (JES).


 On June 17, 2020,
On June 17, 2020, 



 Capitalizing on tiny defects can improve electrodes for lithium-ion batteries, new research suggests.
Capitalizing on tiny defects can improve electrodes for lithium-ion batteries, new research suggests.