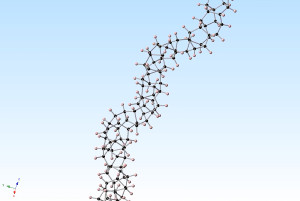
The core of the nanothreads is a long, thin strand of carbon atoms arranged just like the fundamental unit of a diamond’s structure.
Credit: John Badding Lab, Penn State University
A team of scientists have recently discovered how to produce ultra-thin “diamond nanothreads.” These nanothreads, which construct a structure more than 20,000 times smaller than average human hair, are expected to yield extraordinary properties. The new nanothreads will be stronger and stiffer than current nanotubes, and they will also be light in weight.
This means creating the potential for more fuel efficient vehicles, and even fictional-sounding endeavors – such as a “space elevator.”
This from Carnegie Science:
The team—led by John Badding, a chemistry professor at Penn State University and his student Thomas Fitzgibbons—used a specialized large volume high pressure device to compress benzene up to 200,000 atmospheres, at these enormous pressures, benzene spontaneously polymerizes into a long, thin strands of carbon atoms arranged just like the fundamental unit of diamond’s structure—hexagonal rings of carbon atoms bonded together, but in chains rather than the full three-dimensional diamond lattice.