Recent safety concerns with lithium-ion batteries exploding in devices such as the Samsung Galaxy Note 7 phone and hoverboards have many energy researchers looking into this phenomenon for a better understanding of how batteries function when stressed.
Recent safety concerns with lithium-ion batteries exploding in devices such as the Samsung Galaxy Note 7 phone and hoverboards have many energy researchers looking into this phenomenon for a better understanding of how batteries function when stressed.
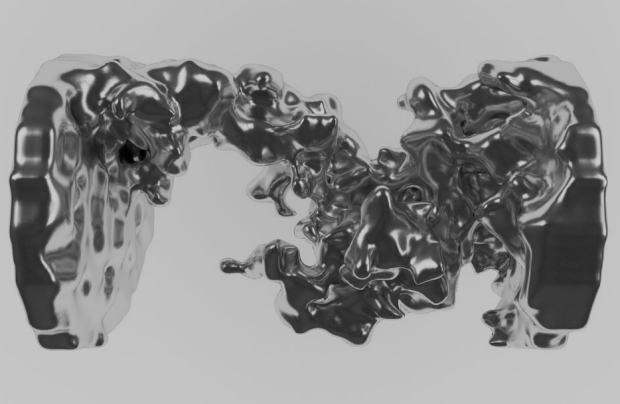
A new open access paper published in the Journal of The Electrochemical Society provides some insight into these safety hazards associated with the Li-ion battery by taking a look inside the battery as it is overworked and overcharged.
Overcharging or overheating Li-ion batteries causes the materials inside to breakdown and produce bubbles of oxygen, carbon dioxide, and other gases. As more of these gases are produced, they begin to buildup and cause the battery to swell. That swelling can lead to explosion.
“The battery can either pillow a small amount and keep operating, pillow a lot and cease operation, or keep generating gas and rupture the cell, which can be accompanied by an explosion or fire,” Toby Bond, co-author of the paper, told New Scientist.


 Twenty-sixteen marked the 25th anniversary of the commercialization of the lithium-ion battery. Since Sony’s move to commercialize the technology in 1991, the clunky electronics that were made possible by the development of the transistor have become sleek, portable devices that play an integral role in our daily lives – thanks in large part to the Li-ion battery.
Twenty-sixteen marked the 25th anniversary of the commercialization of the lithium-ion battery. Since Sony’s move to commercialize the technology in 1991, the clunky electronics that were made possible by the development of the transistor have become sleek, portable devices that play an integral role in our daily lives – thanks in large part to the Li-ion battery.
 In 2005, the number of electric vehicles on the road could be measured in the hundreds. Over the years, researchers have made technological leaps in the field of EVs. Now, we’ve exceeded a global threshold of
In 2005, the number of electric vehicles on the road could be measured in the hundreds. Over the years, researchers have made technological leaps in the field of EVs. Now, we’ve exceeded a global threshold of 
 Lithium-air batteries are viewed by many as a potential next-generation technology in energy storage. With the highest theoretical energy density of all battery devices, Li-air could revolutionize everything from electric vehicles to large-scale grid storage. However, the relatively young technology has a few barriers to overcome before it can be applied. A new study published in the
Lithium-air batteries are viewed by many as a potential next-generation technology in energy storage. With the highest theoretical energy density of all battery devices, Li-air could revolutionize everything from electric vehicles to large-scale grid storage. However, the relatively young technology has a few barriers to overcome before it can be applied. A new study published in the