 ECS teamed up with Amazon to bring ECS members Amazon Catalyst at ECS. ECS members were able to interact with one of the world’s largest companies and potentially be awarded a grant to tackle a number of different challenges.
ECS teamed up with Amazon to bring ECS members Amazon Catalyst at ECS. ECS members were able to interact with one of the world’s largest companies and potentially be awarded a grant to tackle a number of different challenges.
Through the catalyst program, ECS and Amazon looked for solutions that make life easier, healthier, more sustainable, more enjoyable, or more satisfying. The Amazon Catalyst at ECS serves as a prime vehicle for change. Applicants did not need to be established in their field – they just needed a good solution and the passion to carry it out. Amazon Catalyst committed up to $100,000 to help fund the selected ideas.
“Catalyst is about creating a community of people interested in world changing ideas and providing seed funding for them to get that idea underway,” says H.B. Siegel who runs Amazon’s Dept of Ideas. “We are pleased to recognize with grants a few amazing ideas from the many submitted during The Electrochemical Society’s 2018 Catalyst event and look forward to hear about their progress in the coming months.”
The Amazon Catalyst at ECS program was announced by HB Siegel, Amazon’s Director of Engineering in the Department of Ideas, at the 233rd ECS Meeting in Seattle, WA. The top proposals were selected from a pool of 79 submissions. The winners were announced at AiMES 2018 in Cancun, Mexico during the plenary session on Monday, October 1, 2018. Award recipients will provide final presentations to Amazon in Seattle, WA and at the 236th ECS Meeting in Atlanta, GA.
ECS would like to thank Amazon for the partnership that created this unique opportunity for ECS members.
Join ECS and Amazon Catalyst in congratulation the Amazon Catalyst at ECS award recipients:
Mohammadreza Nazemi
Proposal: Using Nanotechnology for Electrosynthesis of Nitrogen-based Fertilizer under Ambient Conditions
Awarded: $25,000
Rajib Das
Proposal: Carbon Catalysts for Cost-Efficient Hydrogen Production in PEM Electolyzers
Awarded: $25,000
Jennifer Schaefer and Peng He
Proposal: Magnesium-Polysulfide Flow Batteries
Awarded: $36,000
Sampath Kommandur and Aravindh Rajan
Proposal: Active Control of Heat Flow in Quantum Computing Applications through Piezoelectric Induced Mechanical Strain
Awarded: $1,600
Winning proposals
Using Nanotechnology for Electrosynthesis of Nitrogen-based Fertilizer under Ambient Conditions
By Mohammadreza Nazemi
Sustainable ammonia production is vital for global population growth due to ammonia’s wide use as a fertilizer in agriculture. The global production of ammonia was 146 million tons in 2015 and is estimated to increase by 40% in 2050. Additionally, ammonia, as a carbon neutral liquid fuel, can be utilized for the development of a clean transportation sector. Ammonia can be a promising substitute for hydrogen as a combustion fuel with superior advantages in terms of energy density, ease of liquefaction, and high hydrogen content (i.e., 17.6 wt%).
Conventional ammonia synthesis mainly relies on the Haber-Bosch process, which converts nitrogen and hydrogen to ammonia at high operating pressures (150-250 bar) and temperatures (350-550oC) over iron-based catalysts. The extreme condition requirements for this process necessitate high-cost demands for centralized infrastructure that should be coupled with the global distribution system. Additionally, this process consumes 3-5% of the global natural gas supply, 60% of the global hydrogen production, and emits 450 million metric tons of CO2 annually.
As the cost of renewably derived (e.g., solar and wind) electricity continues to decrease given the rapid progress in technology and economies of scale, there is a growing interest for ammonia electrosynthesis from nitrogen and water under ambient conditions. This approach can provide an alternative pathway to the Haber-Bosch process for an energy-efficient, clean, and distributed ammonia synthesis.
Electrification of ammonia synthesis in a scale requires an effective electrocatalyst that converts dinitrogen to ammonia with a high yield and efficiency. To date, most studies have shown low electrocatalytic efficiency for ammonia production mainly due to the high energy required for N≡N cleavage and to the competition with the hydrogen evolution reaction (HER). Using molten salt systems and electrochemical lithium cycling strategy result in higher ammonia yield and faradaic efficiency; however, they are not energetically efficient and require high temperatures. Developing an efficient heterogeneous electrocatalyst to remarkably increase the rate of ammonia production through an energy-efficient and environmentally-friendly technique is imperative in energy and agriculture-based industries.
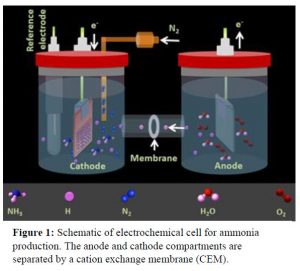 Gold (Au) has been known as one of the best catalysts for the electrochemical NH3 production through an associative mechanism where the breaking of the triple bond of N2 and the hydrogenation of the N atoms occur simultaneously. The selectivity of N2 molecules on the surface of nanocatalysts has been demonstrated to be one of the major challenges in electrochemical NH3 synthesis.
Gold (Au) has been known as one of the best catalysts for the electrochemical NH3 production through an associative mechanism where the breaking of the triple bond of N2 and the hydrogenation of the N atoms occur simultaneously. The selectivity of N2 molecules on the surface of nanocatalysts has been demonstrated to be one of the major challenges in electrochemical NH3 synthesis.
Recently we showed that the nanoscale confinement of N2 near the electrocatalyst’s surface enhances the conversion of N2 to NH3 remarkably using hollow Au nanocages (AuHNCs) [1]. Our reported ammonia yield rate and efficiency are greater than the highest values currently reported in the literature in aqueous solution under ambient conditions.
Electrochemical dinitrogen fixation experiments are conducted in H-type cells where anodic and cathodic compartments are separated by a proton conductive cation exchange membrane (Figure 1). On one side (anode) water is oxidized to produce oxygen gas and protons (H+). The protons produced at the anode are transported through the proton conductive membrane to the other compartment where supplied N2 gas and protons will produce NH3. This electrochemical ammonia generation system is fairly cheap and easy to operate with low maintenance demands.
Although at first glance, it is thought that using Au is not economically feasible for the electrosynthesis of ammonia on a scale, due to the nanoscale nature of the particles, our calculation revealed that the cost of the material used for synthesis is only 8% of the price of ammonia ($800 per ton of ammonia) the farmers currently purchase in the market.
It is hypothesized that the presence of silver in the cavity and increasing the pore size of AuHNCs decrease the rate of electrosynthesis of ammonia. I plan to optimize the pore size and density in the walls of AuHNCs in order to enhance the rate of electro-conversion of N2 to NH3. This can be accomplished by tuning the peak localized surface plasmon resonance (LSPR) of Au nanoparticles. I will study the interconnectedness between the peak LSPR, the presence of Ag in the interior surface of hollow Au nanostructures, the pore size/density, and the active surface area in order to boost the rate of electrochemical NH3 synthesis. This study will elucidate the trade-off between the silver content in the interior cavity of AuHNCs, the peak LSPR of plasmonic nanoparticles, and pore size on the rate of electrochemical ammonia production under ambient conditions.
It has been demonstrated that electrochemical N2 reduction for NH3 production using Pd nanoparticles requires lower overpotential and results in higher electrocatalytic efficiency for NH3 compared with Au nanoparticles. I plan to synthesize hollow Pd nanocages and determine its electrocatalytic efficiency for NH3 production. The results are further complemented by surface-enhanced Raman spectroscopy (SERS) and 1H NMR to monitor the trace amount of NH3 over the course of the experiment and elucidate the possible differences in the reaction mechanism for electrosynthesis of NH3 on Pd and Au surfaces.
The outcome of this work leads to empirical structure-activity trends for ammonia synthesis by an array of hollow plasmonic nanocatalysts with various types (i.e., hollow sphere, hollow cage) and tunable plasmonic properties. I will divide the proposed project into three main categories:
I. Nanoparticle synthesis, characterization, and electrode preparation including deposition of nanoparticle on the substrate using Langmuir-Blodgett technique. (3 months)
II. Electrochemical Testing using various synthesized nanoparticles at different applied potentials in N2 and Ar saturated electrolyte and determine the ammonia yield rate and faradaic efficiency. (6 months)
III. Spectroscopy including SERS and 1H NMR to qualitatively and quantitatively specify the trace amount of ammonia over the course of the experiment. (3 months) (this part can be partially overlapped with part II as well)
Carbon Catalysts for Cost-Efficient Hydrogen Production in PEM Electrolyzers
By Rajib Das
The team of Catalytic Energy Inc. targets development of a novel, earth abundant, low cost carbon catalyst as a replacement for the scarce, expensive platinum catalyst presently used for hydrogen production in water-splitting electrolyzers, which can enable widespread deployment of hydrogen technologies and renewable energy sources.
Replacement of the platinum with carbon-based materials will lead to system cost reductions of approximately 15%. Given platinum’s scarcity, however, its increased demand through wide-spread integration of grid scale renewable energy storage and fuel cell transportation sector, will only increase its proportion of system costs making the proposed development of platinum free electrodes a critical, enabling technology.
Catalytic Energy’s solution will provide benefits for the companies, generating hydrogen for fueling stations, large scale energy storage, enrichment of natural gas and industrial purposes (steel refinement, turbine cooling with hydrogen at power plants, chemical fuel production and others).
As world-wide energy consumption and concern about fossil fuel generated greenhouse gas increases, the development of carbon neutral energy sources remains one of the major challenges facing mankind. Renewable energy sources such as solar, wind and wave energy can, in-principle, satisfy the worldwide energy need, but the sporadic nature of these sources and the asynchronicity between supply and demand remains a major technical obstacle. An efficient energy storage and recovery scheme has emerged in recent years. This consists of hydrogen production from water via electrolysis, thus storing the energy in the form of high energy density, hydrogen chemical fuel, followed by recovery of the electricity and water through high efficiency fuel cells. Hydrogen fuel is of course also portable and thus useful for clean vehicular power applications. The problem in this scheme is that state of the art electrodes in fuel cells and proton exchange membrane (PEM) acidic electrolyzers use precious metal catalysts. Principally, platinum and its alloys for cathode side of the PEM electrolyzer. The scarcity and expense of these metals is prohibitive for their wide-spread deployment. Therefore, alternative, non-precious metal catalysts are actively sought.
The team has recently made the breakthrough discovery that by exposure of the single-walled carbon nanotubes (CNTs), and other low cost layered graphitic carbons (LGCs), to acidic intercalants, in combination with low voltage electrochemical cycling, the materials develop hydrogen evolution activity in acidic aqueous media that initiate at near zero overpotential and is on par with platinum (Pt).1 These results encouraged the further development and testing of the CNT catalyst in a PEM water splitting 2 electrolyzer prototype. Once activated, the CNT cathode demonstrated 1 A/cm2 of HER current at 1.64 V, comparable to the commercial Pt-loaded cathode. While the carbon nanotubes are not intrinsically scarce, the single wall nanotubes used in our electrodes remain relatively expensive. The next step is to implement activated low cost layered graphitic carbons into PEM electrolyzer prototype.
1. R. K. Das et al. ACS Nano, 8, 8447 (2014)
We propose to develop highly efficient, low-cost hydrogen evolution electrodes comprised entirely of activated layered graphitic carbons as Pt replacement for PEM electrolyzer. We will compare the performance of our novel HER electrode with that of the commercial Pt-loaded electrode in the PEM electrolyzer prototype.
Replacement of the platinum loading in commercial electrodes at platinum’s present cost and utilization rates with earth-abundant carbon-based materials will lead to system cost reductions of approximately 15%. Given platinum’s scarcity, however, its increased demand through wide-spread integration of grid scale renewable energy storage and fuel cell transportation sector, will only increase its proportion of system costs making the proposed development of Pt free electrodes a critical, enabling technology.
Companies producing hydrogen for hydrogen fueling stations; Renewable energy suppliers needing to overcome intermittency; Industries that use hydrogen (float glass production, semiconductor manufacturing, steel refinement, turbine cooling in power plants, chemical fuel production etc.), Fossil fuel suppliers upgrading crude oil and enriching natural gas.
The customer will be component manufacturers for PEM electrolyzers: 3M, Dupont, Gore, Freudenberg. Manufacturers of PEM electrolyzers: ProtonOnSite/NEL, Hydrogenics, ITM Power, Giner. Hydrogen fueling station developers: ProtonOnSite/NEL, Plug Power, Nuvera.
At the present rate of population growth global energy consumption will double by 2050 reaching ~ 32 TW. Implementation of zero-carbon emission, renewable energy is essential for humankind’s survival. The intermittency of renewable energy sources like solar, wind and wave energy however remains one of the major obstacles to their large-scale implementation and requires development of appropriate energy storage solutions. High energy density hydrogen fuel is commonly considered the most promising means to store energy supplied from renewable sources. Proton exchange membrane (PEM) water electrolysis presents a sustainable way for the efficient, zero-carbon emission production of high purity and high pressure hydrogen. Presently, platinum remains the best known catalyst for the hydrogen evolution reaction in acidic pH conditions. The scarcity and expense of the platinum is prohibitive for wide-spread deployment of PEM electrolyzer technology. If existing Pt reserves were devoted to operation of 500 million fuel cells vehicles (less than half the vehicles worldwide), it is estimated that all known Pt reserves 3 would be consumed within 15 years even with 50% recycling. Alternative, non-precious metal catalysts are key to the widespread deployment of these technologies.
Despite extensive research on decreasing Pt loading in PEM electrolyzer cathode, there are no low cost hydrogen evolution catalysts that have demonstrated: 1) high activity on par with Pt at commercially relevant current values tested in PEM electrolyzers; 2) high stability on par with Pt in PEM electrolyzers. Our new carbon nanotube-based catalyst has demonstrated such performance. Going one step further – demonstrating such performance for other low cost carbonaceous materials in PEM electrolysis would facilitate widespread deployment of hydrogen generation technologies.
PEM electrolyzers operate in a highly corrosive acidic environment. Apart from our demonstration there are no non-precious metal catalysts, which have shown the necessary combination of high catalytic activity and stability in these severely acidic conditions.
Research efforts on lowering the cost of hydrogen evolution catalysts were twofold: 1) decreasing Pt loading to hundreds of microgram per cm2;2 2) finding alternative, non-precious metal catalysts.3, 4 So far no low cost, non-precious metal catalysts demonstrated commercially relevant activity and stability in PEM electrolyzers. Our unique all-carbon nanotube hydrogen evolution catalyst outperforms all other reported state-of-the-art non-precious metal catalysts in activity and stability in PEM electrolysis.1
1. R. K. Das et al. ACS Nano, 8, 8447 (2014)
2. M. Carmo et.al. Int. J. Hydrogen Energy, 38, 4901 (2013)
3. C. D. Giovanni et al. ACS. Catal. 6, 2626 (2016).
4. J. W. Desmond Ng et al. Chem. Sus. Chem. 8, 3512 (2015)
We have demonstrated exceptional performance of our carbon nanotube hydrogen evolution catalyst in PEM electrolyzers on par with that of the commercial Pt electrode. Scalability of our solution was demonstrated in going from a 1cm2 to a 25 cm2 PEM electrolyzer prototype. While the carbon nanotubes are not intrinsically scarce, the single wall nanotubes used in our electrodes remain relatively expensive. We have also found the HER activity in select forms of other low-cost layered graphitic carbons (LGCs).1 We will screen LGCs that show catalytic activity/stability on par with the CNTs to use these and/or, if necessary, exploit mixtures of LGCs with CNTs to minimize the use of the latter. Our plan 4 is to implement low cost carbon electrodes into PEM electrolyzer prototypes and compare their performance with commercial Pt electrode.
Magnesium-Polysulfied Flow Batteries
By Jennifer Schaefer and Peng He
(This proposal was written in the form of a press release announcing the successful completion of the project in the future)
The Schaefer Research Group today announces the demonstration of magnesium-polysulfide flow batteries operating at enhanced rates, a project supported with $36,000 by Amazon Catalyst at ECS. Magnesium-sulfur batteries were known to be a potentially low cost, large-scale energy storage solution, but previously had been operated only at unpractically slow charge/discharge rates. The Schaefer Research Group increased power and capacity through new electrolyte formulations and adoption of the scalable flow configuration. Implementation of low cost, large-scale energy storage will enable further penetration of renewably generated electricity and a more resilient electric grid.
“Magnesium-sulfur batteries are based on widely abundant materials and thus are a desirable chemistry for high capacity energy storage units, but the research community has been using expensive device formats and reporting cycling performance at exceedingly low rates,” said Jennifer Schaefer, Principal Investigator of the Schaefer Research Group.
“Today’s announcement highlights our efforts to propel the technology forward to competitive operating conditions.”
Schaefer Research Group graduate student Peng He has successfully demonstrated rechargeable batteries with magnesium metal anodes and flowable polysulfide cathodes that perform at higher power densities relevant for grid-scale storage. Electrolytes including high donor number solvents were leveraged to accelerate reaction kinetics. Specially designed membranes, created by the Schaefer Research Group through support by the National Science Foundation, were employed to prevent polysulfide crossover to the anode, enabling long battery lifetimes.
Typical magnesium-sulfur battery cathodes employ expensive, high surface area carbon supports with low sulfur areal loadings, equating to high electrolyte to sulfur ratios and low overall cell energy densities and power densities. The Schaefer Research Group identified that reducing the electrolyte to sulfur ratio in this configuration results in plummeting energy storage capacity. Overall, this widely used research platform is not suitable as a high capacity storage solution.
The Schaefer Research Group leveraged new electrolyte formulations to enable higher performance magnesium-polysulfide batteries for electrical energy storage. Lithium-polysulfide flow batteries have been the topic of active investigation for over 5 years, but the price of lithium worldwide is escalating. Magnesium is substantially more cost-effective than lithium and also less chemically reactive. High donor number solvents such as sulfoxides and sulfones, known to alter polysulfide speciation and reaction rates, are chemically compatible with magnesium metal. The Schaefer Research Group recently demonstrated that reversible magnesium anodes are possible with sulfone-containing electrolytes. In contrast, lithium metal batteries with these high dielectric constant solvents require costly inorganic anode protection layers. Novel electrolyte formulations were used directly in the magnesium-polysulfide flow cell without additional protection layers to achieve the desired result.
The Amazon Catalyst at ECS project was completed in one year. Funding supported procurement of project supplies and a partial graduate student stipend (0.75 persons). Key project milestones included demonstration of enhanced charge rate capability in a conventional magnesium-sulfur platform at three months, demonstration of the magnesium-polysulfide flow cell at six months, and long-term cycling of the magnesium-polysulfide flow cells at project completion. Deliverables at the end of the grant period included photographs and electrochemical cycling data for magnesium-polysulfide flow cells containing varying electrolyte types and operated under varying conditions; all deliverables will be available to the public in an open access format.
About Schaefer Research Group
Schaefer Research Group is led by Prof. Jennifer L. Schaefer of the Department of Chemical and Biomolecular Engineering at the University of Notre Dame. Electrochemical energy storage solutions are the main focus of the group. To learn more, please visit www.schaeferresearch.com.
Active Control of Heat Flow in Quantum Computing Applications through Piezoelectric-Induced Mechanical Strain
By Sampath Kommandur and Aravindh Rajan
There are very few problems that computers cannot solve. However, we are fast reaching computing powers that can be physically expected from the classical computer. Researchers are racing towards new architectures that promise computing powers far greater than that of the classical computer; such as quantum computing and neuromorphic computing for Artificial Intelligence. While these two systems are governed by different phenomena, they do have one unifying challenge: heat. Quantum computers require to be cooled to low temperatures to function. Memristors, a key component of neuromorphic computing memory, requires intense and active thermal management. We propose a novel idea that allows for the active control of heat using a voltage bias. It will make good insulators better for quantum computers and will allow neuromorphic computers to self-correct its memory by redirecting heat flow. These problems necessitate rigorous and thorough research on actively controlling heat flow.
The problem of heat dissipation in quantum computers and maintaining high temperature gradients in memristors set the property constraints for materials in these systems. While there are several research thrusts to achieve these property values, the question on how to relax these constraints has been asked fewer times. We believe that our project is timely, will greatly relieve the constraints researchers are trying to work within right now, and thereby, accelerate progress towards building efficient quantum computers and AI. We are certain that the success of this project will be a crucial cog in the forthcoming computer revolution.
Current technologies focused on manipulating heat flow primarily employ materials that exhibit metal-insulator transitions. Such materials undergo structural changes from a conducting metal to an insulator at a transition temperature, thus conducting heat differently either side of the transition temperature, rendering them effective heat flow manipulators. However, for these materials to be efficient, the operating temperature of the device must be close to the transition temperature. Since the transition temperature is not easily tunable, the solution involves developing and studying new materials that have desirable transition temperatures which often is an inhibitor. Furthermore, as temperature and heat are strongly coupled, the ability to actively control heat flow can be limited.
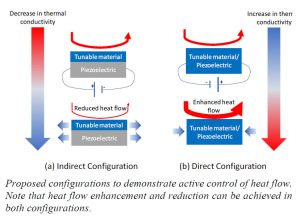 Our proposed solution for active control of heat flow involves altering the thermal conductivity of a material by means of mechanical strain. Stretching (compressing) a material leads to an increase (decrease) in the spacing between the atoms, which results in a decrease (increase) in thermal conductivity of the material. Our proposed approach involves achieving the required strain by applying an electrical voltage, referred to as the piezoelectric effect. We propose to achieve this using two different configurations – an indirect scheme and a direct scheme. The mechanisms to achieve this are clearly depicted in the graphic provided. Briefly, in the indirect configuration the tunable material is deposited on top of a stock piezoelectric material and they experience mechanical strain together. In the direct configuration, the tunable material is also the piezoelectric material. The piezoelectric material in the indirect configuration will be commercially available PVDF and active control will be demonstrated on different tunable materials. In the indirect configuration, block copolymers will be used as the tunable-cum-piezoelectric material. In both these configurations, control of heat flow can be achieved by means of an applied electrical voltage.
Our proposed solution for active control of heat flow involves altering the thermal conductivity of a material by means of mechanical strain. Stretching (compressing) a material leads to an increase (decrease) in the spacing between the atoms, which results in a decrease (increase) in thermal conductivity of the material. Our proposed approach involves achieving the required strain by applying an electrical voltage, referred to as the piezoelectric effect. We propose to achieve this using two different configurations – an indirect scheme and a direct scheme. The mechanisms to achieve this are clearly depicted in the graphic provided. Briefly, in the indirect configuration the tunable material is deposited on top of a stock piezoelectric material and they experience mechanical strain together. In the direct configuration, the tunable material is also the piezoelectric material. The piezoelectric material in the indirect configuration will be commercially available PVDF and active control will be demonstrated on different tunable materials. In the indirect configuration, block copolymers will be used as the tunable-cum-piezoelectric material. In both these configurations, control of heat flow can be achieved by means of an applied electrical voltage.
The active control of heat flow using mechanical strain has only been studied theoretically using detailed atomic simulations. Experimental demonstration of this method has been very limited. However, the results of theoretical studies have been encouraging with thermal conductivity changes upwards of 30% routinely observed in materials like graphene. In some cases, a rectification of 66% has been predicted from just 6% strain. In our proposed study, we plan to use commercially available polymers as piezoelectrics, and the tunable materials will be commercially used metals, semiconductors, and polymers. Since all materials that are being proposed are commercially available, the project and its results are easily scalable and integratable.
Research on the project will span a period of six months and would require a funding of $1,600 for thorough completion. A brief breakdown of the cost estimates is presented below. The cost estimates for cleanroom and Heat Lab are calculated based on standard equipment fees at Georgia Tech, whereas the material cost is based on the price of the proposed materials and polymers.
Research Activity Estimated Cost
| Clean room charges for sample preparation and characterization | $600 |
| Material cost (Silicon wafer substrates, polymers | $600 |
| Heat Lab charges for thermal metrology | $400 |
The primary deliverable at the end of the project will be the successful demonstration of tunable thermal conductivity using piezoelectric effect in two configurations – indirect and direct. The project will involve demonstrating the active control of heat flow by measuring thermal conductivity in the presence and absence of mechanical strain in multiple material sets. Thermal conductivity measurements will be performed across a broader temperature range (-60 oC to 100 oC) to demonstrate the active control at different temperatures. The novel sample geometry and measurement set-up will also be a deliverable so that it can be used for testing future materials. The project will be completed by achieving the following milestones.
| Task | Period | Milestone Description |
| 1 | Months 1-2 | Direct configuration: Sample preparation and thermal measurements |
| 2 | Months 3-4 | Indirect configuration: Sample preparation and thermal measurements |
| 3 | Month 5-6 | Measurements across temperature range -60 to 100 oC. |
The project will be carried out by two senior graduate students at the Scalable Thermal Energy Engineering Lab at Georgia Institute of Technology, Sampath Kommandur and Aravindh Rajan. The members of the team bring together knowledge from diverse research areas including thermal metrology at micro and nanoscale, polymer synthesis and characterization, and thermo-electrochemical energy conversion technologies.
Both members of the team are also associated with Heat Lab at Georgia Tech, which is a center for thermal metrology, catering to the needs of students and external users. Both team members are also experienced users of microfabrication and characterization tools, and one team member is currently a leading member of the Heat Lab and will champion the efforts towards thermal measurements. This makes the team very well positioned to achieve the proposed goals of the project.


